New Delhi, March 7 (IANS): Researchers at the S. N. Bose National Centre for Basic Sciences, Kolkata, an autonomous institute under the Department of Science and Technology (DST), have discovered an efficient, less energy-intensive, and environmentally friendly method to synthesize hydrogen peroxide (H2O2) using sunlight. This breakthrough offers a sustainable alternative to conventional industrial production methods that are energy-intensive and generate hazardous by-products.
Hydrogen peroxide is a vital chemical used across various industries, including environmental disinfection, chemical synthesis, paper bleaching, and fuel cells. With the increasing global demand driven by heightened disinfection awareness and the rise in hospital-acquired infections, scientists have been seeking greener and more cost-effective production strategies.
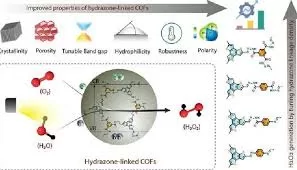
The research team developed a series of covalent organic frameworks (COFs) with high water affinity by carefully controlling the hydrazone linkage density. COFs are a novel class of porous and ordered polymers known for their tunable catalytic properties and efficient light-harvesting abilities. These materials have shown great promise as photocatalysts for H2O2 generation.
Currently, over 95% of industrial hydrogen peroxide is produced using the anthraquinone oxidation process, which is not only expensive but also produces hazardous by-products. The researchers’ innovative approach involves the use of hydrazone-linked COFs to facilitate two key reactions: the water oxidation reaction (WOR) and the oxygen reduction reaction (ORR), both of which are essential for photocatalytic H2O2 synthesis.
The study revealed that the hydrazone-linked COFs exhibit remarkable photocatalytic performance when exposed to a 40 W blue LED, producing significant amounts of hydrogen peroxide. Notably, the method also demonstrated excellent efficiency under direct sunlight, outperforming most organic photocatalysts under similar conditions. This discovery underscores a clean and sustainable pathway for large-scale hydrogen peroxide production.
Furthermore, the researchers found that using an aqueous benzyl alcohol solution (90% water, 10% benzyl alcohol) not only enhanced H2O2 generation but also prevented its degradation. The team believes that this strategy could pave the way for the development of a continuous-flow reactor, enabling industrial-scale production with minimal environmental impact.
“This method provides a sustainable approach for hydrogen peroxide synthesis and represents a significant step towards clean energy solutions,” the researchers noted. The study’s findings highlight the potential for a seamless transition from laboratory research to industrial application, benefiting both the environment and the chemical industry.
Disclaimer: This article is based on research findings and is intended for informational purposes only. The practical implementation and commercialization of this method may require further studies and regulatory approvals.