Our immune system is a vigilant guardian, perpetually on alert to detect and eliminate pathogens and cancer cells. Diseased cells present antigens on their surface as markers for the immune system, enabling the identification and elimination of threats. Understanding the intricate processes of antigen presentation is critical for advancements in immunology and therapeutic development. A groundbreaking study by a German research team, as reported in the journal Angewandte Chemie, introduces an innovative method to analyze these processes in real time.
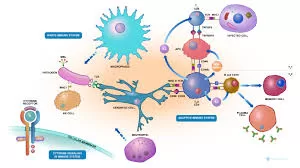
Within our cells, both endogenous and foreign proteins are continuously broken down into tiny pieces and transported into the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP). The ER, a complex network of channels enclosed by a membrane, is the site where the supramolecular peptide loading complex (PLC) oversees the loading of major histocompatibility complex class I (MHC I) molecules with antigenic peptides. These peptides are then presented on the cell surface for immune surveillance. Peptides from normal endogenous proteins usually remain unnoticed by the immune system unless there is a misdirected autoimmune reaction.
Despite significant progress, the mechanistic principles of antigen translocation, dynamic PLC assembly, and the interaction between PLC subunits in peptide-MHC complex quality control remain largely unknown. To delve deeper into these mechanisms, Ralph Wieneke, Robert Tampé, and their team at the University of Frankfurt am Main have developed a photostimulated antigen release system. This innovative system allows for the precise study of antigen flux by releasing antigens from a “caged” state using light.
The team utilized a peptide derived from an HIV antigen as their model. They bound the epitope (antigen segment) to biotin, which was then linked to a large protein called streptavidin, effectively shielding the epitope from recognition by TAP. The linker contained a group that could be split apart by light. When exposed to UV light, the peptide epitope was immediately released from its “cage,” enabling recognition by TAP, transportation across the ER membrane, and loading onto MHC I by the PLC.
The use of light stimulation offers significant advantages: it can be precisely dosed, applied at specific times and locations, and is noninvasive, allowing for experiments in living cells. This method proved versatile, as demonstrated by tracking antigen transport by TAP in the ER membrane of a human lymphoma cell line.
“Our aim is to use the light-activated system to follow the antigen processing pathway through different cellular compartments and gain an understanding of the kinetics of various immunological processes in vivo,” Wieneke and Tampé stated.
This novel approach promises to shed light on the intricate processes of antigen presentation, providing valuable insights that could inform the development of new immunotherapies and enhance our understanding of immune system functioning. The ability to control and observe antigen processing with such precision marks a significant step forward in immunological research.