Situation at a glance
On 7 March 2023, the National IHR Focal Point (NFP) for Germany notified WHO of five cases of iatrogenic botulism in individuals who underwent medical procedures with the injection of botulinum neurotoxin type A (BoNT/A) in health institutions in Türkiye. As of 17 March 2023, a total of 71 cases were reported in four countries in the European Region, mainly linked to two hospitals in different locations in Türkiye.
The products used for the treatment were seized and taken for examination and evaluation by the Turkish Medicines and Medical Devices Agency. No new symptomatic cases have been reported since 8 March 2023.
Outbreaks of botulism are very rare and can be associated with a natural, accidental, or potentially deliberate source of infection.
Description of the situation
On 7 March 2023, the National IHR Focal Point (NFP) for Germany notified WHO of five cases of iatrogenic botulism in individuals who underwent medical procedures with the injection of botulinum neurotoxin type A (BoNT/A) in health institutions in Türkiye. As of 17 March 2023, a total of 71 clinical botulism cases were reported from Türkiye (53 cases), Germany (16 cases), Austria (one case) and Switzerland (one case) linked to the aforementioned medical interventions performed in Türkiye between 22 February and 1 March 2023.
All cases are adults; most cases are middle-aged women. Among the 69 cases for which treatment location information is known, two private hospitals in two locations in Türkiye were identified, with 66 cases linked to one hospital and three cases to another hospital.
The clinical presentation of the cases ranged from mild to severe. Clinical signs of BoNT intoxication were observed (including fatigue, headache, blurred and/or double vision, dizziness, ptosis, dysphagia, dyspnea, neck weakness, generalized muscle weakness, and swollen tongue). Several cases were hospitalized. Some cases were treated with botulinum antitoxin. At least five cases were admitted to intensive care units. There are no reported deaths.
Investigations carried out by Turkish authorities reported that licensed BoNT products were administered for a different purpose other than for which the products were approved (off-label use). The relevant departments of both hospitals had their activities suspended on 1 March 2023, and investigations have been launched against the parties involved. The products used for the treatment were seized and taken for examination and evaluation by the Turkish Medicines and Medical Devices Agency. No new symptomatic cases have been reported since 8 March 2023, which was the date of symptom onset for the last reported case.
Public health response
The Turkish Ministry of Health is working with the Türkish Medicines and Medical Devices Agency and the Turkish National Poison Solidarity Center to investigate this event. The Turkish General Directorate of Public Health, Department of Communicable Diseases and Early Warning. is conducting an epidemiological investigation with provincial health centres and related departments. The relevant units of the Ministry of Health and the Turkish Police Service urgently inspected the two hospitals mentioned above. The investigation is currently ongoing to determine if the cause of the intoxication is due to product quality/safety, medical error, or an overdose.
WHO is collaborating with the European Centre for Disease Prevention and Control (ECDC), and the affected IHR State Parties, regarding investigation, risk assessment, information sharing and response for this event.
The Turkish Medicines and Medical Devices Agency published an announcement on its website on 10 March 2023. Similarly, the ECDC announced the current situation regarding this event in their publicly-available Communicable Disease Threats Report on 10 March 2023, and a news story was published on the ECDC website on 14 March 2023.
WHO risk assessment
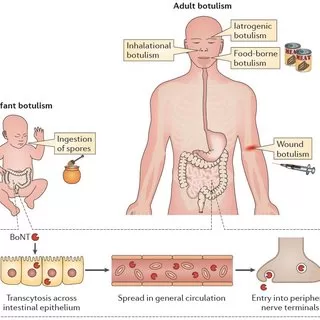
Botulinum neurotoxins are dangerous toxins produced by several species of the genus Clostridium (e.g., C. botulinum, C. baratii, C. butyricum, and C. sporogenes) under anaerobic conditions. Botulinum neurotoxins are one of the most lethal substances known and occur in eight serotypes (A to H). Botulinum neurotoxins block nerve functions and can lead to muscular, including respiratory, paralysis. Human botulism is a naturally occurring rare disease and may refer to foodborne botulism, infant botulism, wound botulism, and inhalation botulism.
Clostridium botulinum culture is used to produce pharmaceutical botulinum toxin-containing products. These pharmaceutical products employ the purified and heavily diluted botulinum neurotoxin (type A or type B, depending on the indication) injected for clinical use for the treatment of more than 20 neurological and non-neurological disorders, as well as for cosmetic use. Treatment is administered in the medical setting, tailored according to the needs of the patient and desired treatment effect.
The symptoms of botulism can be very severe, requiring intensive-care treatment as well as the administration of specific anti-toxins. Even when such treatments are available, severe botulism cases require supportive treatment, including mechanical ventilation. Complete recovery usually takes weeks to months.
This current event is possibly linked to a specific procedure and/or a medical product in at least two healthcare settings. Further investigations are ongoing regarding case ascertainment and notification. The investigation remains open for other possible hypotheses of intoxication. The respective departments in the two clinics where the procedures were undertaken have been closed since 1 March, with no further cases reported.
There are several clinical indications for the use of botulinum neurotoxin; however, its off-label use in bariatric cases has increased in many countries. Depending on the product used, a warning of toxic effects may be insufficiently worded, and the risks not adequately addressed in the patient consent process.
WHO advice
Botulism outbreaks are rare, but outbreaks require rapid recognition to identify the source of the disease, distinguish between types of outbreaks (natural, accidental or potentially deliberate), prevent additional cases, and effectively administer treatment to affected patients.
Successful treatment depends significantly on early diagnosis and the rapid administration of the botulinum antitoxins. Iatrogenic botulism should be suspected if a patient has recently received botulinum neurotoxin, and clinicians should remain mindful of the risk of systemic botulism with BoNT therapy. The diagnostic detection of BoNT/A in patients suffering from iatrogenic botulism might be highly challenging due to the extremely low dose of toxin present and the potentially suboptimal timepoint of serum sampling. So far, very few detection methods have been shown to be validated on clinical specimens with a sensitivity better than the classical mouse bioassay, which is still regarded as a reference for BoNT detection in clinical samples. At this time, it cannot be confirmed if the reported cases of botulism intoxication are due to product quality/safety, medical error, or an overdose.
Although the source of the outbreak may have been removed, WHO Member States need to remain vigilant. It is therefore critically important for the IHR State Parties to identify any additional possible case, to establish the cause, and to determine if the product used in the procedure could be substandard or falsified. Investigation of these cases will assist in rapid containment at the disease source to prevent additional possible cases.
WHO does not recommend any travel and/or trade restrictions to Türkiye based on the information available for this event.
Further information
- WHO Health topics: Botulism, available at https://www.who.int/news-room/fact-sheets/detail/botulism
- Turkish Medicines and Medical Devices Agency announcement on 10 March 2023 (1), available at: https://titck.gov.tr/duyuru/botox-preparatlari-hakkinda-duyuru-27122018173114(link is external)
- European Centre for Disease Prevention and Control Communicable Disease Threats Report Week 10, 5–11 March 2023, available at https://www.ecdc.europa.eu/sites/default/files/documents/Communicable-Disease-Threats-Report-10-Mar-2023.pdf(link is external)
- European Centre for Disease Prevention and Control, News story,14 March 2023, Botulism cases in Europe following medical interventions with botulinum neurotoxin, available at: https://www.ecdc.europa.eu/en/news-events/botulism-cases-europe-following-medical-interventions-botulinum-neurotoxin(link is external)
- Iatrogenic Botulism Outbreak in Egypt due to a Counterfeit Botulinum Toxin A Preparation–A Descriptive Series of Patient Features and Outcome, available at https://onlinelibrary.wiley.com/doi/epdf/10.1111/bcpt.13048(link is external)
Citable reference: World Health Organization (24 March 2023). Disease Outbreak News; Iatrogenic Botulism– European Region. Available at https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON450