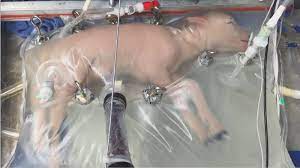
The research team from the Children’s Hospital of Philadelphia (CHOP) that conducted the ‘artificial womb’ experiment in 2017 is now seeking approval from the Food and Drug Administration (FDA) to conduct human clinical trials. The experiment involved keeping a developing lamb alive for 28 days in a sterile bag filled with fluid, providing it with amniotic fluid, medicine, and oxygen through tubes connected to umbilical cord tissue. This successful experiment demonstrated positive growth in lung, GI tract, and brain development in the lamb.
The device being tested, called the Extra-uterine Environment for Newborn Development (EXTEND), aims to simulate some elements of a natural womb, with the goal of increasing survival rates and improving outcomes for extremely premature babies. It is important to note that the technology is not intended to support development from conception to birth.
Alan Flake, a foetal surgeon at CHOP leading the effort, expressed the hope that if successful, the majority of pregnancies predicted to be at risk for extreme prematurity could be delivered early onto their system rather than being delivered prematurely onto a ventilator.
Several members of the CHOP team, including Alan Flake, have joined a startup company called Vitara Biomedical in Philadelphia. The company has raised $100 million to further develop the EXTEND system.
The FDA’s meeting with independent advisers will focus on discussing regulatory and ethical considerations, as well as outlining what human trials for the technology might entail. Kelly Werner, a bioethicist and neonatologist at Columbia University Medical Center in New York City, noted that this development is an exciting step and has been eagerly anticipated by clinicians working with premature babies. They will closely follow the progress of this technology.










