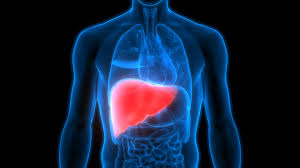
New research from Yale University suggests that targeting hormones could potentially reduce the risk of fatty liver disease, specifically metabolic associated dysfunction steatotic disease (MASLD), in individuals with a genetic predisposition. The study, published in Cellular and Molecular Gastroenterology and Hepatology, delves into the role of the PNPLA3I148M gene variant, a significant contributor to genetically driven MASLD.
Fatty liver disease, characterized by excess fat accumulation in the liver, can lead to severe health complications, including inflammation, scarring, and even liver cancer. With obesity and type 2 diabetes on the rise, the prevalence of non-alcohol-related fatty liver disease, affecting approximately 30% of the global population, is expected to increase.
Researchers focused on the PNPLA3I148M gene variant, which has been implicated in altered fat distribution both within and outside the liver. Using genetically modified mice carrying this variant, they observed that a high-sugar diet resulted in less fat accumulation in adipose tissue (body fat) but more fat buildup in the liver compared to mice without the variant.
Further investigation revealed that catecholamines, hormones like adrenaline and noradrenaline, play a role in this process. When mice with the PNPLA3I148M variant were treated with a drug mimicking these hormones, they exhibited increased breakdown of adipose fat, leading to further liver fat accumulation.
However, when these mice were treated with beta blockers, such as propranolol and acipimox, which block catecholamine receptors, they showed a reduction in liver fat buildup. This suggests that blocking catecholamine action outside the liver could potentially mitigate fatty liver disease risk in individuals with the PNPLA3I148M variant.
“This was a clue that there might be some form of adipose tissue dysfunction to be discovered,” said Dr. Daniel Vatner, assistant professor of medicine (endocrinology) at Yale School of Medicine and senior author of the study.
While the results are promising, Dr. Vatner cautioned that translating these findings to human treatments requires further research. “Often in mice, it’s a little easier to see the response than in humans,” he explained, citing factors like alcohol consumption, diabetes, and rapid weight loss that can influence liver fat accumulation in humans.
Dr. Vatner is currently seeking funding to investigate whether these findings can be replicated in human patients and whether beta blockers could serve as a viable treatment option for individuals with a genetic predisposition to MASLD.
“What I hope is that 10 years down the road, we know enough about this work that we can say we’re developing new treatment strategies based on this science,” he concluded.
Disclaimer: This article is based on research findings in mice and should not be interpreted as medical advice. Further research is necessary to determine the efficacy and safety of beta blockers for treating fatty liver disease in humans. Individuals with fatty liver disease or concerns about their liver health should consult with a healthcare professional for personalized guidance and treatment.