Hong Kong – A groundbreaking study from the University of Hong Kong’s LKS Faculty of Medicine (HKUMed) has identified the mitochondrial protein Chchd10 as a crucial regulator of adipose tissue, offering potential new strategies for combating obesity and related metabolic diseases. The findings, published in the journal Advanced Science, shed light on the complex molecular mechanisms governing fat tissue health.
Obesity, a global health crisis, is closely linked to metabolic disorders like type 2 diabetes and cardiovascular diseases. The research team focused on understanding how adipose tissue remodeling, the process by which fat cells increase in number and size in response to excess energy intake, can become imbalanced, leading to obesity.
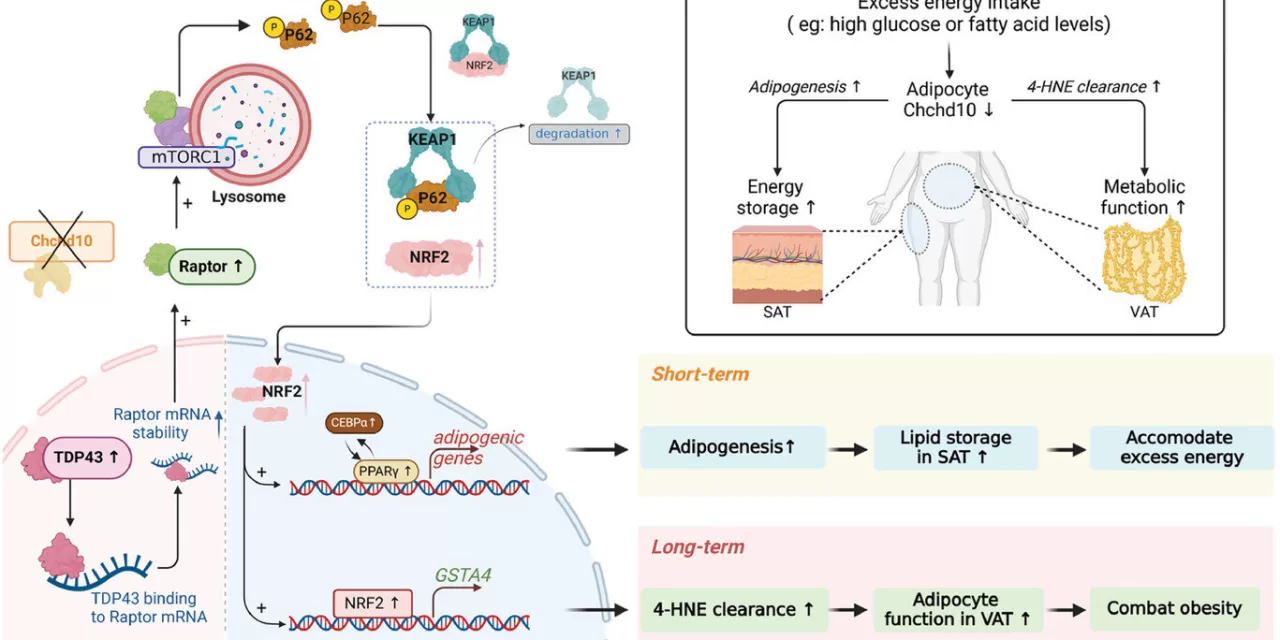
The study utilized high-fat diet (HFD)-induced mice, both wild-type and adipose tissue-specific Chchd10-deficient, to investigate the protein’s role. Researchers observed that in HFD-induced mice, Chchd10 levels significantly decreased in white adipose tissue, the primary site for energy storage. This reduction accelerated subcutaneous fat formation, allowing for greater energy storage during short-term high-fat diets.
Conversely, the absence of Chchd10 led to increased levels of GSTA4, a protein vital for clearing harmful lipid peroxidation products in visceral fat tissue, preventing cellular dysfunction after prolonged high-fat diet consumption. The study revealed that Chchd10 downregulation activates the NRF2 signaling pathway, which promotes both subcutaneous fat formation and GSTA4 expression.
“This study provides valuable insights into the molecular pathways involved in regulating adipose tissue homeostasis and their implications for obesity management,” said Professor Ruby Hoo Lai-chong, Associate Professor in the Department of Pharmacology and Pharmacy, HKUMed. “By targeting Chchd10 and its associated pathways, new therapeutic strategies can be developed to combat diet-induced obesity and improve metabolic health. The research underscores the potential interventions targeting metabolic dysfunctions in specific adipose depots.”
Notably, in mice experiencing weight gain from a high-fat diet, knocking out Chchd10 in adipose tissue significantly reduced visceral fat mass accumulation, suggesting that regulating Chchd10 levels could be a promising therapeutic avenue for obesity treatment.
In a related study, also published in Advanced Science, Professor Hoo’s team explored the role of tissue-resident memory T (TRM) cells in type 1 diabetes development. This research highlighted how TRM cells, through the fatty acid-binding protein 4 (FABP4) and the inflammatory chemokine CXCL10, contribute to the recruitment of cytotoxic T cells to pancreatic islets, driving the onset of type 1 diabetes. The team found that genetic deletion of FABP4 or depletion of TRM cells delayed diabetes onset and reduced cytotoxic T cell recruitment in non-obese diabetic mice, indicating that targeting FABP4 could be a potential therapeutic strategy.
The research findings on Chchd10 provide a strong foundation for future studies aimed at developing targeted therapies for obesity and related metabolic disorders.
Disclaimer: This article is based on scientific research published in Advanced Science. While the findings are promising, further research and clinical trials are necessary to validate these results in humans. This information should not be interpreted as medical advice. Always consult with a healthcare professional before making any changes to your diet,1 exercise, or treatment plan.