January 22, 2025
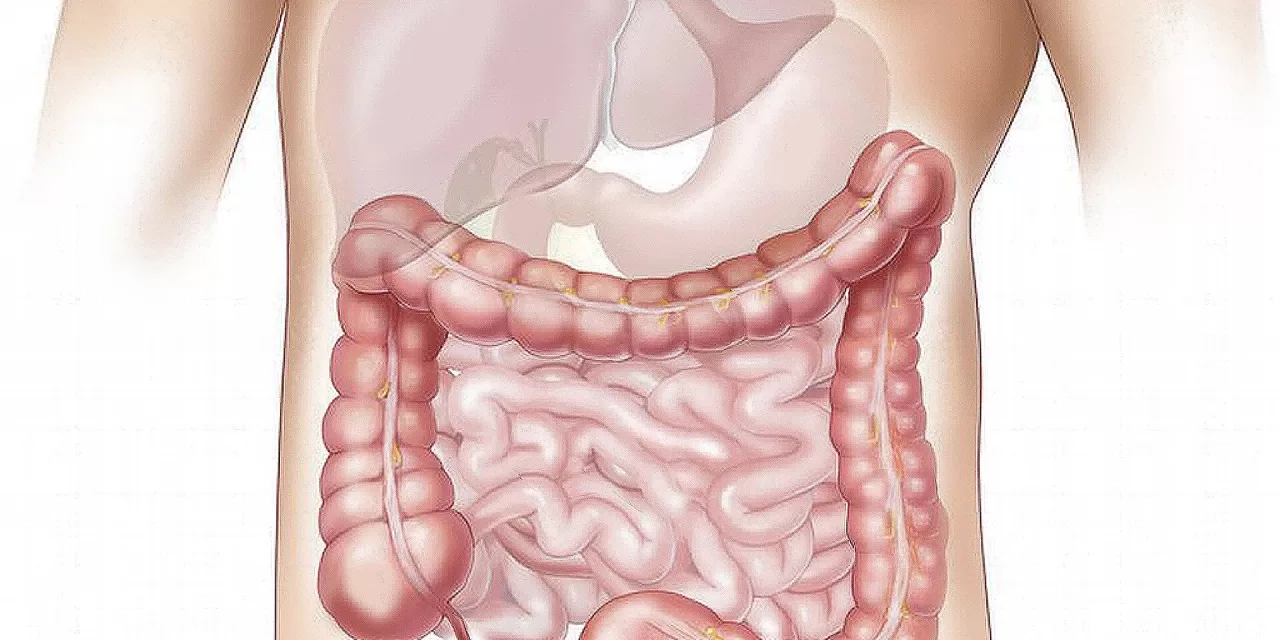
In a groundbreaking study, researchers from Harvard Medical School have uncovered a critical link between gut bacteria and depression, shedding light on the complex interplay between the gut microbiome, inflammation, and mental health. The findings, published in the Journal of the American Chemical Society, highlight how a specific bacterium, Morganella morganii, may influence major depressive disorder (MDD) through inflammatory mechanisms.
Unpacking the Gut-Depression Connection
The human gut microbiome has long been known to affect various aspects of health, including mental well-being. However, identifying the specific bacterial players and mechanisms involved in mental disorders has proven challenging. Researchers have now pinpointed how M. morganii produces an abnormal molecule in the gut that triggers an inflammatory immune response.
This molecule arises when M. morganii incorporates a contaminant called diethanolamine (DEA), often found in industrial, agricultural, and consumer products, into its biological processes. The resulting abnormal molecule stimulates the release of cytokines—immune proteins linked to inflammation—particularly interleukin-6 (IL-6), a protein previously associated with depression and inflammatory diseases such as type 2 diabetes and inflammatory bowel disease (IBD).
Inflammation and Depression: A Molecular Link
The study bolsters the hypothesis that chronic inflammation contributes to the development of depression. “This research provides a coherent story from M. morganii at the beginning to depression at the end,” said senior author Jon Clardy, the Christopher T. Walsh, Ph.D. Professor of Biological Chemistry and Molecular Pharmacology at HMS.
By revealing the molecular pathways connecting gut bacteria to inflammation and mental health, the study offers a potential new target for diagnosing or treating depression. DEA, the contaminant at the heart of this discovery, may join the growing list of biomarkers for MDD.
Rethinking Depression as an Immune-Driven Disorder
The findings suggest that some cases of depression could be classified as autoinflammatory or autoimmune diseases, potentially treatable with immune-modulating drugs. “This study strengthens arguments that MDD could, at least in part, be an immune-driven disorder,” Clardy said.
The research also opens new avenues for studying how other gut bacteria influence immunity and human biology. “Now that we know what to look for, we can begin surveying other bacteria to uncover similar mechanisms,” added Clardy.
Collaborative Research to Advance Understanding
The discovery was made possible by combining expertise in bacterial chemistry and microbiome research from the labs of Clardy and Ramnik Xavier, the Kurt J. Isselbacher Professor of Medicine at Massachusetts General Hospital. Their collaborative work has previously revealed how gut bacteria like Akkermansia muciniphila and Ruminococcus gnavus influence inflammation and disease outcomes.
Future Directions
While the study offers compelling evidence of M. morganii‘s role in depression, further research is needed to confirm the bacterium’s influence as a definitive cause of MDD and to estimate its impact across the population.
“This discovery is just the beginning,” Clardy emphasized. “It provides a roadmap for investigating how gut bacteria impact mental health and other aspects of human biology.”
With its potential to revolutionize the understanding and treatment of depression, this research underscores the importance of exploring the gut-brain connection in depth.
Citation:
Sunghee Bang et al., “Unusual Phospholipids from Morganella morganii Linked to Depression,” Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.4c15158