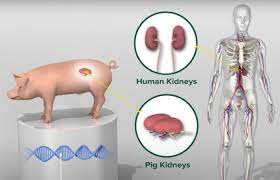
A significant milestone in xenotransplantation has been achieved by a Chinese multidisciplinary team, who successfully transplanted a gene-modified pig liver into a brain-dead human recipient. The groundbreaking procedure, detailed in a peer-reviewed publication in the journal Nature, marks the first documented instance of a genetically altered pig liver functioning within a human host.
During a 10-day observation period, the transplanted porcine liver demonstrated crucial functions, including the production of bile and porcine albumin, maintenance of stable blood flow, and the absence of rejection signs. This accomplishment opens up potential avenues for utilizing pig livers as a temporary bridge to human liver transplants, particularly for patients facing acute liver failure.
“This is an important experiment,” stated Dr. Rafael Matesanz, founder of Spain’s National Transplant Organization, “as it opens up the possibility of using a porcine liver as a temporary replacement of the diseased liver until a human liver can be obtained for the definitive transplant.”
The donor pig underwent genetic modifications to inactivate genes responsible for rejection and incorporate human transgenes for enhanced compatibility. Researchers, led by Dr. Ke-Feng Dou from Xijing Hospital, confirmed the successful genetic alterations before the transplant. The surgical technique employed by the team was described by Dr. Peter Friend, a professor of transplantation at the University of Oxford, as “very elegant,” minimizing disruption to the recipient’s existing liver anatomy.
Post-transplantation, the team meticulously monitored the liver’s function, blood flow, and immune responses. Immunosuppressant drugs were used to manage immune reactions. The liver exhibited normal alanine aminotransferase levels, with a transient increase in aspartate aminotransferase on the first postoperative day. C-reactive protein and procalcitonin levels, initially elevated, quickly declined.
While the preliminary results are promising, the study’s duration was limited to 10 days at the request of the recipient’s family. This constraint prevented long-term monitoring of the liver’s function. Furthermore, the team primarily assessed basic liver functions, highlighting the need for further research to evaluate the full spectrum of liver capabilities and long-term outcomes.
“I found the work very relevant, but we have to be cautious,” cautioned Dr. Iván Fernández Vega, professor of pathological anatomy at the University of Oviedo. He emphasized the necessity for larger-scale studies involving living recipients to validate the procedure’s safety, efficacy, and reproducibility.
This achievement joins other recent advancements in xenotransplantation, including pig-to-human heart and kidney transplants performed by US research teams. These developments underscore the field’s rapid progress and its potential to address the critical shortage of human organ donors.
Disclaimer: This news article is based on the provided information and should not be taken as medical advice. Xenotransplantation is a complex and evolving field. Further research and clinical trials are required to fully understand the long-term safety and efficacy of these procedures. The information presented here reflects the findings of a single study and may not be representative of all future outcomes.