A groundbreaking study led by researchers at UMass Chan Medical School demonstrates potential for gene therapy to treat maple syrup urine disease (MSUD).
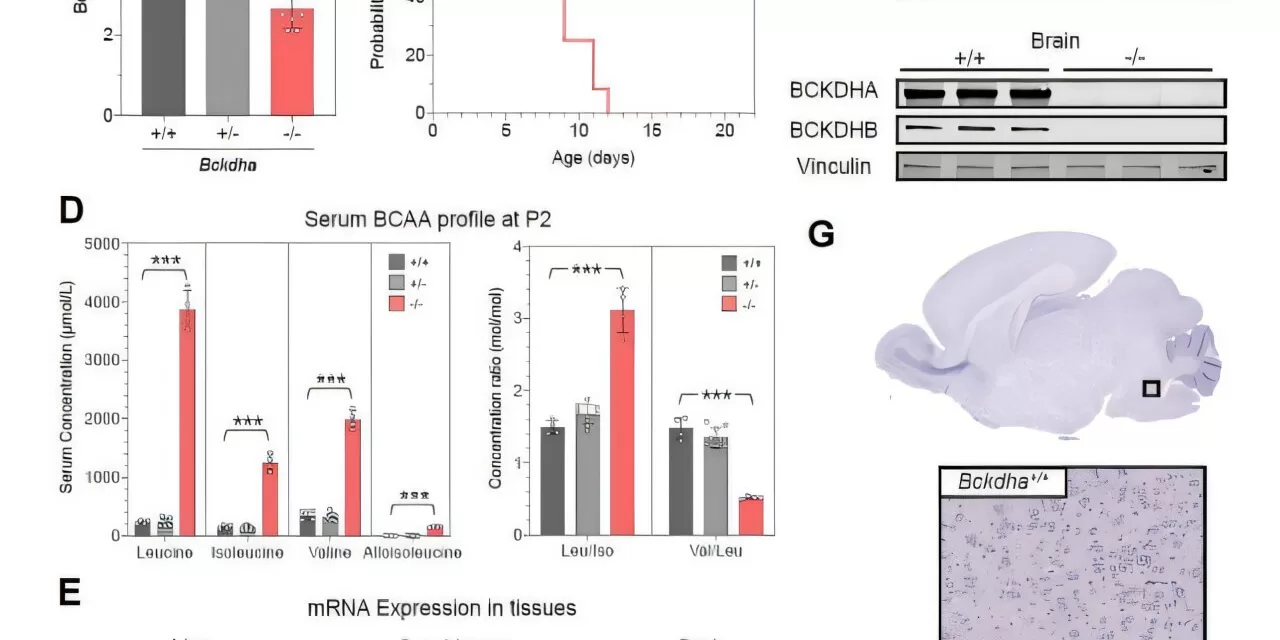
A newly developed gene therapy designed to correct the genetic mutation responsible for MSUD has shown remarkable success in preclinical trials. The study, published in Science Translational Medicine, revealed that the therapy prevented newborn death, normalized growth, restored coordinated gene expression, and stabilized biomarkers in both mice and a calf.
Significant Breakthrough in Gene Therapy
Dr. Dan Wang, assistant professor of genetic & cellular medicine and co-principal investigator of the study, expressed optimism about the findings.
“Simply put, we believe the gene therapy demonstrated in both animal species, especially in the cow, very well showcases the therapeutic potential for MSUD, in part because the diseased cow, without treatment, has a very similar metabolic profile as the patients,” Dr. Wang stated.
Other key investigators include Dr. Heather Gray-Edwards, Dr. Guangping Gao, and Dr. Kevin Strauss, who collaborated on the research.
Understanding Maple Syrup Urine Disease
MSUD is a rare genetic disorder that prevents the body from properly breaking down certain proteins, leading to toxic substance accumulation. This can result in severe neurological complications and brain damage if not managed through a strict prescription diet or liver transplant. The disease occurs in approximately one in 197,714 live births but is significantly more common in specific populations, such as Mennonites in Lancaster County, Pennsylvania.
MSUD is caused by mutations in the BCKDHA, BCKDHB, or DBT genes. The newly developed therapy utilizes a recombinant adeno-associated virus serotype 9 vector to deliver a gene replacement to critical organs, including the liver, muscle, heart, and brain.
Encouraging Results from Animal Models
The study’s use of a calf model was particularly significant, as it provided valuable insight into how the therapy might perform in human patients. Data from the calf study helped researchers understand pharmacokinetics, muscle and brain tissue effects, and long-term treatment durability.
Dr. Strauss recounted the early stages of the project, explaining how researchers and physicians convened on an Iowa cattle farm in 2018 to begin testing the therapy. “In the years that followed, physicians at the Clinic for Special Children worked intently with scientists and veterinarians from UMass Chan Medical School to achieve that goal, drawing their inspiration from the hopes and struggles of the MSUD community. For people worldwide living with MSUD, this signifies major progress on the path to a brighter future.”
Looking Ahead: Clinical Trials and FDA Approval
Following these promising results, researchers are now working towards advancing this gene therapy to human clinical trials. Plans are underway for a Phase I/II study with the U.S. Food and Drug Administration to evaluate safety and efficacy in human patients.
The potential of this gene therapy to offer an alternative to lifelong dietary restrictions or liver transplantation marks a significant milestone in the treatment of MSUD. While further research and trials are necessary, the study provides hope for individuals affected by this rare metabolic disorder.
Disclaimer: This article reports on preclinical research, and the gene therapy discussed is not yet approved for human use. Individuals with MSUD should continue to follow their prescribed treatment plans and consult with their healthcare providers before considering any new therapies.