In groundbreaking research, scientists have shown that gene therapy could potentially revolutionize the treatment of hypophosphatasia (HPP), a rare inherited disorder that causes abnormal bone development and early tooth loss. Current treatments for this condition require frequent enzyme replacement injections, but researchers believe a single-dose gene therapy might offer lifelong relief.
For the past decade, the only effective treatment for HPP has been enzyme replacement therapy using asfotase alfa, a mineral-targeted form of the missing enzyme tissue-nonspecific alkaline phosphatase (TNAP). This therapy, approved by the FDA, has been a lifesaver for many children who would have otherwise faced early death due to severe bone malformation. However, the treatment requires injections three to six times a week, which some patients find invasive and difficult to maintain.
Dr. José Luis Millán, a leading researcher at Sanford Burnham Prebys and professor in the Human Genetics Program, described the enzyme replacement therapy as “a tremendous success” but acknowledged its limitations. “It’s been a lifesaving treatment, but it’s also very invasive. Some patients discontinue treatment due to reactions from frequent injections,” said Millán.
HPP, also known as soft bone disease, has varying degrees of severity. The most severe form affects approximately one in 100,000 live births and can cause life-threatening complications. While milder forms primarily impact adults by increasing the risk of fractures, the severe form of HPP often results in significant bone deformities and early death in infants.
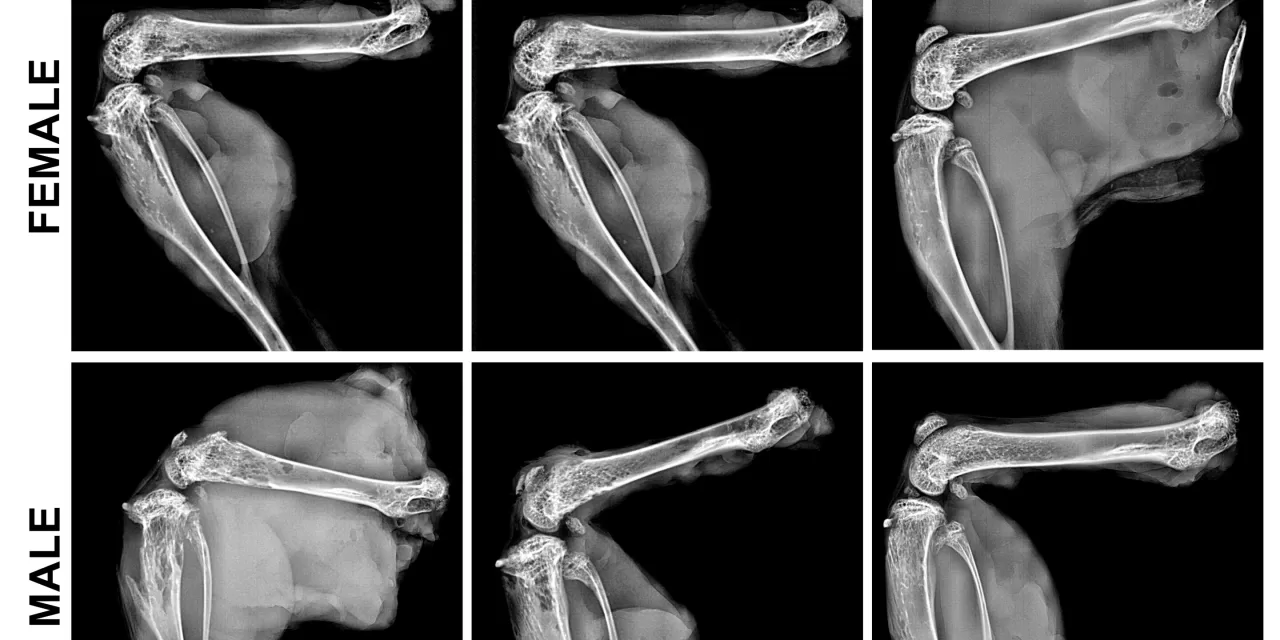
A major shift in the future of treatment may come from gene therapy, which could offer a permanent solution with a single injection. In a recent study published in the Journal of Bone and Mineral Research, Dr. Millán and his team presented their findings on AAV8-TNAP-D10, a gene therapy that delivers the missing enzyme directly to the body via a viral vector. This innovative approach has demonstrated promising preclinical results, indicating it could reverse the bone and dental deformities caused by HPP.
“We believe the next evolution in treating HPP will be a gene therapy in which a single injected dose will provide a lifelong treatment for patients,” said Millán.
The study, which also involved researchers from Japan’s Nippon Medical School, focused on testing various doses of the gene therapy on mice. Their findings suggest that the therapy is both safe and effective in treating both early and late-onset forms of HPP. The research included an unexpected discovery: the therapy appeared to be more effective in female mice, achieving bone and tooth improvements with a lower dose compared to male mice. However, Millán emphasized that this phenomenon is unique to mice and is not expected to occur in humans.
With successful preclinical data in hand, the next step is to move towards clinical trials. Millán and his collaborators are now seeking partnerships with companies to advance AAV8-TNAP-D10 into human trials, with hopes that it will offer a less invasive, long-lasting alternative to enzyme replacement therapy.
Though the potential of this gene therapy is exciting, Dr. Millán remains focused on the long-term needs of HPP patients. While enzyme replacement and gene therapies can address skeletal mineralization, the missing TNAP enzyme is also found in the brain, liver, kidneys, and immune system. “We need to anticipate long-term problems before they happen so we can be prepared to help patients with HPP throughout their lives,” he said.
As the field moves forward, researchers continue to explore the broader applications of gene therapy for HPP, with hopes of improving the quality of life for those affected by this rare and challenging disease.
Disclaimer: The findings presented in this article are based on preclinical research and are not yet available as a treatment option for humans. Clinical trials are necessary to further evaluate the safety and effectiveness of the AAV8-TNAP-D10 gene therapy in patients with hypophosphatasia.