The WHO Director-General transmits the report of the fourth meeting of the International Health Regulations (2005) (IHR) Emergency Committee regarding the multi-country outbreak of monkeypox (mpox), held on Thursday, 9 February 2023 from 12:00 to 17:00 CET.
Following a series of consultations with global experts, WHO recommends a new preferred term “mpox” as a synonym for monkeypox. Both names will be used simultaneously for one year while the term “monkeypox” is phased out. This report uses the term “mpox” (for more information see here).
The Emergency Committee acknowledged the progress made in the global response to the multi-country outbreak of mpox and the further decline in the number of reported cases since the last meeting. The Committee observed that a few countries continued to see a sustained incidence of illness; the Committee is also of the view that underreported detection and under-reporting of confirmed cases of illness in other regions is likely. Therefore, the Committee considered various options to sustain attention and resources to control the outbreak and advised maintaining the Public Health Emergency of International Concern (PHEIC), while beginning to consider plans to integrate mpox prevention, preparedness and response within national surveillance and control programmes, including for HIV and other sexually transmissible infections.
The WHO Director-General expresses his gratitude to the Chair, Members, and Advisers for their advice and concurs with this advice that the event continues to constitute a PHEIC for the reasons detailed in the proceedings of the meeting below and issues revised Temporary Recommendations in relation to this PHEIC, which are presented at the end of this document.
Proceedings of the fourth meeting of the IHR Emergency Committee
The fourth meeting of the IHR Emergency Committee on the multi-country outbreak of mpox was convened by videoconference, with the Chair and Vice-Chair being present in person on the premises of WHO headquarters, Geneva, Switzerland. Thirteen of the fifteen Members and four of the nine Advisors to the Committee participated in the meeting.
In his opening remarks, the WHO Director-General welcomed the Committee, and noted a sustained decline in cases globally, with the majority of cases being reported from the Regions of the Americas. The Director-General also noted the need to sustain efforts for surveillance, prevention and care; vaccinate high-risk populations; improve equitable access to diagnostics, vaccines and treatment for all who need them; and continue to fight stigma and discrimination and ensure respect for human rights. While noting that the continued human-to-human transmission could lead to a resurgence of cases, he concluded that over the longer-term, mpox programmes and services should be integrated into national surveillance and control programmes, including for HIV and other sexually transmitted infections.
The Office of Legal Counsel’s representative briefed the Committee Members and Advisors on their roles, responsibilities, and mandate under the relevant articles of the IHR. The Ethics Officer from the Department of Compliance, Risk Management, and Ethics reminded Members and Advisers of their duty of confidentiality as to the meeting discussions and the work of the Committee, as well as their individual responsibility to disclose to WHO in a timely manner any interests of a personal, professional, financial, intellectual or commercial nature that may give rise to a perceived or direct conflict of interest.
The meeting was handed over to the Chair of the Emergency Committee, Dr Jean-Marie Okwo-Bele, who introduced the objectives of the meeting: to provide views to the WHO Director-General as to whether the multi-country outbreak of mpox continues to constitute a PHEIC, and, if so, to review the proposed Temporary Recommendations to States Parties.
Presentations
Representatives of Brazil updated the Committee on the epidemiological situation in their country and their current response efforts.
The Secretariat provided an update on the epidemiological situation and the current response efforts, with the WHO Regions of Europe and the Americas providing additional regional updates.
The Secretariat informed that the current global risk of the mpox multi-country outbreak is assessed as remaining moderate globally and in four of the WHO regions, reduced from moderate to low in the Region of South-East Asia and remaining low in the Western Pacific Region. Further details can be found in the 15th External situation report. All data are available, and case counts are updated weekly at this link – 2022 Monkeypox Outbreak: Global Trends.
The Secretariat further informed the Committee that its Strategic preparedness, readiness and response plan for monkeypox, and appeal, launched in July 2022 to help guide coordinated public health action to stop the outbreak will come to an end in June 2023, while additional resources are being sought through the WHO’s Health Emergency Appeal in 2023.
The WHO European Region reported that as of 3 February, 43 countries and territories have not detected any new cases in the past three months. While 18 countries and territories continue to report recent local human-to-human transmission, case numbers have decreased significantly. Future risks of outbreaks relate to the ongoing importation, forthcoming mass gatherings, potential reduced vaccination and surveillance, limited access to testing and behaviour change/. To tackle this, the Region is working towards a five-year plan to achieve and sustain the elimination of mpox in all Member States through engagement with affected communities and integrating intervention into the sexual health programs, to be discussed at the Regional Committee in autumn 2023.
The Region of the Americas reported a stable number of cases in the last six weeks, with 200-250 cases per week, and 4% of cases occurring in women. In addition, while the vaccine supply is limited, seven countries have started vaccination. Risk communication and community engagement interventions are being delivered through HIV community-based networks.
After the presentations, Committee Members and Advisors proceeded to engage the Secretariat and the presenting country in a question-and-answer session.
Deliberative session
The Committee reconvened in a closed meeting to examine the questions in relation to whether the event continues to constitute a PHEIC, and if so, to consider the proposed Temporary Recommendations, drafted by the WHO Secretariat in accordance with IHR provisions. The Secretariat provided a presentation on the legal provisions under the IHR in relation to the determination of a PHEIC, and the issuance of Temporary Recommendations.
The Committee acknowledged that further progress was made in reducing the number of cases as well as international transmission, but several concerns persist. These include: ongoing transmission in some regions, such as Central America; insufficient evidence regarding vaccine effectiveness on the individual and population levels and duration of immunity, either disease or vaccination-induced; a potential shift in some countries towards the most marginalized populations who have the least access to prevention measures and treatments; the possibility that behaviour change is not sustained in the long run; and reduced surveillance and lack of reporting of cases to WHO, particularly in countries where the disease is endemic.
On the positive side, the Committee notes that the global risk is assessed as moderate, with two regions having a low risk; that no significant changes in the demographics occurred, although a small number of cases were reported in women in the region of the Americas; that the predominant mode of transmission remains through direct and sexual contact; and that transmission declined in a number of countries prior to the escalation of vaccination programs, concurrently with community engagement activities, acquired immunity after infection amongst those who were at highest risk, and a growing understanding of transmission dynamics. In addition, some regions have started to develop post-emergency plans and began the integration of the response into sexually transmissible disease programs.
Nevertheless, the Committee expressed concerns about the possible resurgence of cases in some regions, due to both the potential seasonal differences in the occurrence of infection and particularly in the context of the resumption of LGBTQ social events and other mass gathering events in the coming months; the lack of access to vaccines and testing capacities; the recurring zoonotic transmission in Africa; the fact that not all countries are receiving the support they need or have structures or systems to respond to mpox, including inadequate support for marginalized groups; and general fatigue among supporting agencies in view of competing priorities and emergencies.
The Committee acknowledged one WHO region’s proposal to develop a five-year elimination strategy and stressed the need for all countries to rapidly develop and continue to implement existing short-term responses to mpox and begin the development of national and regional plans aimed at long-term elimination of human-to-human transmission or control, as appropriate and feasible, with gradual integration into HIV and other sexually transmissible disease programs.
Lastly, noting that mpox transmission still persists in some countries and that there remain important research gaps to optimize the knowledge needed to tame the outbreaks, the Committee advised maintaining PHEIC and provided advice on the draft Temporary recommendations prepared by the Secretariat, with the understanding that such Temporary recommendations may continue to be issued by the WHO Director-General if needed after the future termination of the PHEIC.
Temporary Recommendations issued by the WHO Director-General in relation to the multi-country outbreak of mpox
These proposed Temporary Recommendations continue to support the goal of the Strategic Preparedness, Readiness and Response Plan for Monkeypox 2022–2023 with the aim of stopping the ongoing mpox outbreak and its objectives to interrupt human-to-human transmission, protect the vulnerable, and minimize zoonotic transmission of the virus.
Significant progress has been achieved in ending the ongoing multi-country mpox outbreak with a decline in cases globally. While the previously issued Temporary Recommendations continue to hold, recommendations for areas that continue to represent challenges and emerging areas of work due to lessons learned are emphasized in this document.
These Temporary Recommendations apply to States Parties according to their epidemiological situation, patterns of transmission, and capacities with respect to mpox; they refer to the reality that any State Party may experience importation or local transmission of mpox and some States Parties may also be experiencing zoonotic transmission.
Thus, each State Party should develop a strategy to maintain surveillance and response capacity in the medium to long term while States Parties in a position to support scaling up access to medical countermeasures, including through technology transfer, should continue efforts. With cases in some countries increasingly occurring within communities/individuals experiencing racism and other stigma and discrimination, strategies to reach these groups through risk communication, prevention and treatment are critical. In implementing these Temporary recommendations, States Parties should ensure full respect for the dignity, human rights and fundamental freedoms of persons, in line with the principles set out in Article 3 of the IHR.
The WHO advises States Parties to prepare short and medium to long-term plans for the control of mpox, and to maintain vigilance and response capacity as well as engagement with local communities and key stakeholders, following the WHO relevant guidelines.
States Parties should strengthen action in the following key areas:
- Develop and implement operational plans, including monitoring and evaluation, to set clear targets for stopping human-to-human transmission of mpox in countries currently affected by the outbreak, or mpox control in countries with known animal-to-human transmission. In that regard, State Parties should also consider developing surveillance and control plans that apply to situations where intimate sexual contact is not necessarily the predominant mode of transmission.
- Maintain laboratory-based epidemiological surveillance, including reporting of the minimum dataset of variables defined in the WHO Case Reporting Form. States Parties should continue to share confirmed and probable mpox case reports with WHO through IHR communications in a timely manner. Countries should work towards the elimination of mpox (i.e. interrupting local or community transmission, and taking measures to promptly detect and contain imported outbreaks) where feasible and maintain high-quality indicator-based and event-based surveillance to underpin all such efforts.
- Integrate mpox surveillance, detection, prevention, care and research into innovative HIV and STI prevention and control programmes and services, in order to understand risks of resurgence, detect outbreaks early, reduce barriers to health services, communicate risk, strengthen detection of undiagnosed HIV infection and early and continued antiretroviral treatment, advance clinical care for HIV-mpox co-infection, and address fear, stigma and discrimination in at-risk populations.
- Continue to enhance access to diagnostics, vaccines and therapeutics, including through allocation mechanisms and technology transfer, and subsidy of regional manufacturing to advance global health equity in areas where people may experience barriers to care, including minorities and those in the global south.
- Strengthen and support capacity in resource-limited settings where mpox continues to occur, including for but not limited to One Health and animal health, to better understand and characterize all modes of transmission and respond to outbreaks wherever they occur.
- Implement a strategic and coordinated research agenda to ensure ongoing evidence generation including but not limited to a better understanding of mpox clinical virology, modes of transmission, social determinants for affected groups and clinical disease, particularly in immune-suppressed individuals, and development of countermeasures, including effective behaviour change, rapid diagnostics and next-generation therapeutics and vaccines.
The recommendations below extend or modify those issued on 1 November 2022. In line with the WHO announcement on 28 November 2022, the term monkeypox in reference to the disease has been replaced with mpox throughout this document. WHO documents referenced are current as of 7 February 2023.
Readiness (1): These recommendations are meant to ensure a state of readiness for an outbreak of mpox and apply to ALL States Parties
ADDED: 1.a.0. Prepare a strategy to strengthen capacity in key areas of readiness and response including: 1) develop a short-term mpox elimination or control plan as appropriate to national circumstances 2) ensure that mpox is a nationally notifiable disease, and strengthen epidemiological disease surveillance to support efforts towards mpox elimination or control, as appropriate; 3) Strengthen and leverage innovative HIV and STI programmes and services to integrate mpox surveillance, detection, prevention, care and research; 4) continue to enhance access to diagnostics, vaccines and therapeutics for hard-to-reach and marginalized populations to advance gobal health equity; 5) continue to strengthen capacity in resource limited settings through a One Health approach to better understand and prevent transmission at the animal-human interface; 6). Stimulate, incentivize, fund and support a research agenda to address critical knowledge gaps and ensure ongoing evidence generation.
MODIFIED: 1.a. Develop an elimination or control plan for mpox that encompasses a multi-sectoral and One Health approach, and maintain coordination mechanisms to strengthen all aspects of readiness to stop human-to-human transmission and respond to mpox and undertake an after-action review to identify and build on lessons learned during the outbreak and address residual risk, reduce the threat of future mpox outbreaks and enhance readiness for outbreaks. https://www.who.int/publications/m/item/monkeypox-strategic-preparedness–readiness–and-response-plan-(sprp)
EXTENDED: 1.b. Continue to plan for, and/or implement, interventions to avoid the stigmatization and discrimination against any individual or population group that may be affected by mpox, with the goal of preventing further undetected transmission of monkeypox virus (MPXV). The focus of these interventions should be: to promote voluntary self-reporting and care-seeking behaviour; to support access to vaccines and therapeutics; to facilitate timely access to quality clinical care; to protect the human rights, privacy and dignity of at-risk and affected individuals, and their contacts across all communities. https://www.who.int/publications/i/item/WHO-MPX-RCCE-2022.1
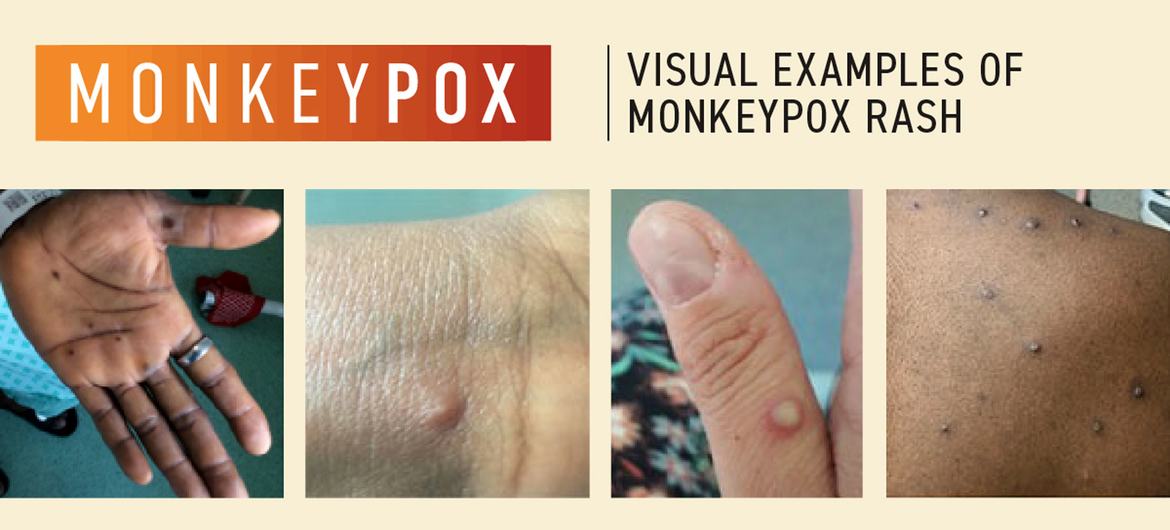
EXTENDED: 1.c. Noting that Clade II MPXV is a sexually transmissible infection, continue to establish and intensify epidemiological disease surveillance, including access to reliable, affordable and accurate diagnostic tests, for illnesses compatible with mpox as part of existing national surveillance systems. For disease surveillance purposes, case definitions for suspected, probable and confirmed cases of mpox should be adopted, as well as the case definition for death related to mpox. https://www.who.int/publications/i/item/WHO-MPX-Surveillance-2022.4
https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1
EXTENDED: 1.d. Continue to intensify the early-detection capacity by raising awareness and training of health workers, including those in primary care, genitourinary and sexual health clinics, urgent care/emergency departments, dental practices, dermatology, paediatrics, HIV services, infectious diseases, maternity services, obstetrics and gynaecology, and other acute care facilities. https://www.who.int/publications/i/item/WHO-MPX-Surveillance-2022.4
EXTENDED: 1.e. Continue to raise awareness about MPXV transmission, related prevention and protective measures, and symptoms and signs of mpox among communities that are currently affected (e.g., importantly, but not exclusively, some communities of gay, bisexual and other men who have sex with men (MSM) or individuals with multiple sexual partners) as well as among other populations that may be at risk (e.g., sex workers, transgender people). https://www.who.int/publications/i/item/WHO-MPX-RCCE-2022.1
MODIFIED: 1.f. Continue to engage key community-based groups, sexual health and civil society networks, including those for persons living with HIV, to listen and understand community perceptions and concerns about mpox and to increase the provisions of reliable and factual information about the disease. https://www.who.int/publications/i/item/WHO-MPX-RCCE-2022.1
MODIFIED: 1.g. Continue to focus risk communication and community support efforts on settings and venues where intimate encounters take place (e.g., gatherings focused on MSM, sex-on-premises venues) as well as channels that may facilitate targeted messaging to affected communities (such as geospatial dating apps for MSM). This includes engaging with and supporting community-led organizations, the organizers of large and smaller scale events, as well as owners and managers of sex on premises venues to promote personal protective measures and risk-reducing behaviour. https://www.who.int/publications/i/item/WHO-MPX-Gatherings-2022.1
MODIFIED: 1.h. Countries with newly detected cases or deaths of mpox should report thee cases to WHO as soon as they are detected. Countries currently experiencing an outbreak should report probable and confirmed cases of mpox, and deaths related to mpox to WHO on a weekly basis, through channels established under the provisions of the IHR, using the minimum data set contained in the WHO Case Report Form (CRF). https://www.who.int/publications/m/item/monkeypox-minimum-dataset-case-reporting-form-(crf) https://www.who.int/publications/i/item/WHO-MPX-Surveillance-2022.4; https://www.who.int/publications/i/item/WHO-MPX-Clinical_CRF-2022.3
EXTENDED: 1.i. Continue to implement all actions necessary to apply or continue applying the set of Temporary Recommendations enumerated under Response (2) below in the event of first-time or renewed detection of one or more suspected, probable or confirmed cases of mpox.
Outbreak response (2) : All States Parties with one or more cases of mpox, regardless of the initial source, or experiencing human-to-human transmission, including in key population groups or communities at high risk of exposure
2.a. Implementing coordinated response
EXTENDED: 2.a.i. Continue to implement response actions with the goal of stopping human-to-human transmission of MPXV, with a priority focus on communities at high risk of exposure, which may differ according to context and may include gay, bisexual and other men who have sex with men (MSM). Those actions include: targeted risk communication and community engagement, case detection, supported isolation of cases and treatment, contact tracing, and targeted immunization for persons at high risk of exposure for mpox. https://www.who.int/publications/m/item/monkeypox-strategic-preparedness–readiness–and-response-plan-(sprp)
EXTENDED: 2.a.ii. Continue to support the empowerment of affected communities and enable and support their leadership in devising, actively contributing to, and monitoring the response to the health risk they are confronting. Extend technical, financial and human resources to the extent possible and maintain mutual accountability on the actions of the affected communities. https://www.who.int/publications/m/item/monkeypox-strategic-preparedness–readiness–and-response-plan-(sprp)
EXTENDED: 2.a.iii. Continue to implement responses with the goal of protecting vulnerable groups (immunosuppressed individuals, children, pregnant women) who may be at increased risk of severe mpox disease. Those actions include: targeted risk communication and community engagement, case detection, supported isolation of cases, contact tracing, and treatment. This may also include targeted immunization which takes into careful consideration the risks and benefits for the individual in shared clinical decision-making. https://www.who.int/publications/m/item/monkeypox-strategic-preparedness–readiness–and-response-plan-(sprp)
2.b. Engaging and protecting communities
MODIFIED: 2.b.i. Continue to raise awareness about how mpox presents and transmits in affected communities, and particularly in hard-to-reach and marginalized populations which may vary by context, and actions that can be taken to reduce risk. Promote the uptake and appropriate use of prevention measures, including supporting equitable access to primary preventive vaccination (PPV) for persons at risk of exposure, and adoption of other informed risk mitigation measures in line with the most recent WHO guidance. https://www.who.int/publications/i/item/WHO-MPX-Gatherings-2022.1
EXTENDED: 2.b.ii. Continue to engage with authorities and event organizers of gatherings (large and small), including those likely to be conducive for encounters of an intimate nature or that may include venues for sex-on-premises, to promote personal protective measures and behaviours, and encourage organizers to apply the WHO-recommended risk-based approach to decision-making regarding the holding of such events. Provide all necessary information for risk communication on personal choices around preventive measures including the role of vaccines, reduction in numbers of partners, and for infection prevention and control including safer sexual practices and regular cleaning of event venues and premises. https://www.who.int/publications/i/item/WHO-MPX-Gatherings-2022.1
MODIFIED: 2.b.iii. Continue to develop and target risk communication and community engagement interventions, including systematic social listening (e.g., through digital platforms) for emerging perceptions, concerns, and spreading of misinformation that might hamper response actions. Co-develop communication materials with communities for greater understanding of evolving evidence-based information. https://www.who.int/publications/i/item/WHO-MPX-RCCE-2022.1
EXTENDED: 2.b.iv. Continue to engage with representatives of affected communities, non-government organizations, elected officials and civil society, and behavioural scientists to advise on approaches and strategies to avoid the stigmatization of any individual or population groups in the implementation of appropriate interventions, so that care seeking behaviour, testing and access to and adoption of preventive measures, and clinical care is timely, and to prevent undetected transmission of MPXV. https://www.who.int/publications/i/item/WHO-MPX-RCCE-2022.1
2.c. Surveillance and public health measures
MODIFIED: 2.c.i. Intensify surveillance for illness compatible with mpox as part of existing national surveillance schemes, including access to timely, reliable, affordable and accurate diagnostic tests. https://www.who.int/publications/i/item/WHO-MPX-Surveillance-2022.4;
EXTENDED: 2.c.ii. Continue to report probable and confirmed cases of mpox, and deaths related to mpox to WHO, on a weekly basis, including through channels established under the IHR (2005), or on a monthly basis if management of mpox has been integrated into HIV or other sexually transmissible programs, including using the minimum data set contained in the WHO Case Report Form (CRF). Mpox (monkeypox) Case investigation form (CIF) and minimum dataset Case reporting form (CRF) (who.int)
https://www.who.int/publications/i/item/WHO-MPX-Surveillance-2022.4; https://www.who.int/publications/i/item/WHO-MPX-Clinical_CRF-2022.3
EXTENDED: 2.c.iii. Continue to strengthen laboratory capacity (including through international specimen referral as needed), and support within-country decentralized access to testing, where possible, for the diagnosis of MPXV infection, and related surveillance, based on the use of nucleic acid amplification testing (NAAT), such as real time or conventional polymerase chain reaction (PCR). https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1
EXTENDED: 2.c.iv. Continue to strengthen genomic sequencing capacities, and international specimens referral capacities as needed, building on existing sequencing capacities worldwide, to determine circulating virus clades and their evolution, and share genetic sequence data through publicly accessible databases. https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1
MODIFIED: 2.c.v. Isolate cases for the duration of the infectious period. Policies related to the isolation of cases should encompass health, psychological, material and essential support to adequate living. Any adjustment of isolation policies late in the isolation period should entail the mitigation of any residual public health risk. Advise persons identified as having suspected or confirmed mpox, during the isolation period on how to minimise the risk of onward transmission of mpox, such as covering lesions and wearing a mask in accordance with the most recent WHO guidance. https://www.who.int/publications/i/item/WHO-MPX-Clinical-and-IPC-2022.1
NOT APPLICABLE: 2.c.vi. Previously merged with 2.c.v
EXTENDED: 2.c.vii. Continue to conduct contact tracing among individuals in contact with anyone who may be a suspected, probable, or confirmed case of mpox, including: contact identification (protected by confidentiality), notification management, and follow-up for 21 days through health monitoring which may be self-directed or supported by public health officers. Policies related to the management of contacts should encompass health, psychological, material and essential support to adequate living. https://www.who.int/publications/i/item/WHO-MPX-Surveillance-2022.4
EXTENDED: 2.c.viii. Continue to consider the targeted use of second- or third-generation smallpox or mpox vaccines (hereafter referred to as vaccine(s)) for post-exposure prophylaxis in contacts, including household, sexual and other contacts of community cases and health workers where there may have been a breach of personal protective equipment (PPE). https://www.who.int/publications/i/item/WHO-MPX-Immunization
EXTENDED: 2.c.ix. Continue to consider the targeted use of vaccines for primary preventive (pre-exposure) vaccination, particularly for persons and communities at high risk of exposure. Persons at highest risk of exposure in the multi-country outbreak are gay, bisexual or other MSM with multiple partners. Others at risk may include individuals with multiple casual sexual partners, sex workers, and those who may be exposed and at risk for more severe disease. Those at risk may also include health workers prone to repeated exposure, laboratory personnel working with orthopoxviruses, and clinical laboratory personnel performing diagnostic testing for mpox.
https://www.who.int/publications/i/item/WHO-MPX-Immunization
EXTENDED: 2.c.x. Continue to convene the National Immunization Technical Advisory Group (NITAG) for any decision about immunization policy and the use of vaccines. Recommendations should be informed by risks-benefits analysis. In all circumstances, vaccinees should be informed of the time required for protective immunity potentially offered by vaccination. https://www.who.int/publications/i/item/WHO-MPX-Immunization
EXTENDED: 2.c.xi. Continue to engage the communities at high risk of exposure in the decision-making process regarding any vaccine roll out. https://www.who.int/publications/i/item/WHO-MPX-Immunization
EXTENDED: 2.c.xii. Continue to undertake thorough risk assessments, prepare for, and rapidly respond to any case or outbreak of mpox in congregate settings. This includes hospitals, prisons, migrant worker residences, or other situations where population density may be high, including facilities for internally displaced persons or refugees. https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox–external-situation-report–13—5-january-2023
2.d. Clinical management and infection prevention and control
EXTENDED: 2.d.i. Establish and continue to use recommended clinical care pathways and protocols for the screening, triage, isolation, testing, and clinical assessment of suspected persons with mpox in all clinical settings where persons with compatible clinical syndromes may present, including but not limited to urgent or primary care, sexual health services and dermatology clinics; provide training to health care providers accordingly and monitor the implementation of those protocols. https://www.who.int/publications/i/item/WHO-MPX-Clinical-and-IPC-2022.1
EXTENDED: 2.d.ii. Establish and continue to implement protocols related to infection prevention and control (IPC) measures in line with the most recent WHO guidance, encompassing engineering and administrative controls and the use of PPE; provide training to health care providers accordingly, and monitor the implementation of those protocols. https://www.who.int/publications/i/item/WHO-MPX-Clinical-and-IPC-2022.1
MODIFIED: 2.d.iii. Continue to provide health and laboratory workers with adequate PPE, as appropriate for health facility and laboratory settings, and provide all personnel with training in the use of PPE. Consider pre-exposure preventive vaccination for health workers as appropriate based on assessed benefits and risks. https://www.who.int/publications/i/item/WHO-MPX-Clinical-and-IPC-2022.1; https://www.who.int/publications/i/item/WHO-MPX-Immunization
EXTENDED: 2.d.iv. Continue to establish, update, and implement evidence-based clinical protocols for the care and management of people with uncomplicated mpox (e.g., keeping lesions clean, pain control, and maintaining adequate hydration and nutrition); and the various manifestations of severe disease; prevention and treatment of acute complications; and monitoring and management of mid- or long-term sequelae, including provision of social and psychological support where needed. Establish mpox case detection and care through integrated approaches with established sexual health and HIV prevention and care services, including through community engagement with civil society organisations. https://www.who.int/publications/i/item/WHO-MPX-Clinical-and-IPC-2022.1
EXTENDED: 2.d.v. Continue to harmonise data collection and report clinical outcomes, using the WHO Global Clinical Platform for mpox. https://www.who.int/tools/global-clinical-platform/monkeypox
2.e. Medical countermeasures research
EXTENDED: 2.e.i. Continue to make all efforts to use existing or new vaccines against mpox within a framework of collaborative clinical efficacy studies, using standardized design methods and data collection tools for clinical and outcome data, to rapidly increase evidence generation on efficacy and safety, collect data on effectiveness of vaccines (e.g., such as comparison of one or two dose vaccine regimens), and conduct vaccine effectiveness studies. https://www.who.int/publications/i/item/WHO-MPX-Immunization
EXTENDED: 2.e.ii. Continue to make all efforts to use existing or new therapeutics and antiviral agents for the treatment of mpox within a framework of collaborative clinical efficacy studies, using standardized design methods and data collection tools for clinical and outcome data, to rapidly increase evidence generation on efficacy and safety. https://www.who.int/publications/i/item/WHO-MPX-Clinical-and-IPC-2022.1
EXTENDED: 2.e.iii. When the use of vaccines and antivirals for mpox in the context of a collaborative research framework is not possible, use under expanded access protocols can be considered, such as the Monitored Emergency Use of Unregistered and Investigational Interventions (MEURI), under certain circumstances, using harmonized data collection for clinical outcomes (such as the WHO Global Clinical Platform for mpox). https://www.who.int/publications/i/item/9789240041745
EXTENDED: 2.e.iv. Continue to encourage, support and facilitate data gathering and priority research in areas of work relevant to mpox, including but not limited to disease transmission and the natural history of disease’ diagnostics and innovative technologies including point-of-care tests, viral kinetics across specimen types and animal diagnostics; behavioural insights research and studies on effectiveness of interventions; exposure risk for health workers and pre- and post-exposure management; research on zoonotic transmission of mpox at the human-animal-environment interface, including, socio-economic and behavioural risk factors, and indications for environmental surveillance in wastewater. https://www.who.int/news-room/events/detail/2022/06/02/default-calendar/who-monkeypox-research–what-are-the-knowledge-gaps-and-priority-research-questions
2.f. Domestic and international travel
EXTENDED: 2.f.i. Continue to adopt and apply the following measures:
- Any individual who is suspected, probable, or confirmed mpox=infected by jurisdictional health authorities should avoid undertaking any travel, including international travel, until they are cleared to do so. Anyone who is unwell should be advised to seek medical attention prior to travel.
- Any individual who has been identified as a contact of a person with mpox, and is therefore subject to health monitoring, should avoid undertaking any travel, including international travel during the health monitoring period, except for contacts for whom pre-departure arrangements to ensure continuity of health monitoring are agreed upon by the health authorities concerned, or, in the case of international travel, between national health authorities.
Exemptions apply for any person with mpox or contact and who may need to undertake travel to seek urgent medical care or flee from life threatening situations, such as conflict or natural disasters.
- https://www.who.int/publications/i/item/WHO-MPX-Surveillance-2022.4 Cross-border workers, who are identified as having been exposed to mpox, and, hence, under health monitoring, can continue their routine daily activities provided that health monitoring is duly coordinated by the jurisdictional health authorities from both/all sides of the border.
EXTENDED: 2.f.ii. Continue to maintain operational channels between health authorities, transportation authorities, and conveyances and points of entry operators to:
- Facilitate international contact tracing in relation to individuals who have developed signs and symptoms compatible with MPXV infection during travel or upon return;
- Provide communication materials at points of entry on signs and symptoms consistent with mpox; infection prevention and control; and on how to seek medical care at the place of destination;
WHO advises against any additional general or targeted international travel-related measures other than those specified in paragraphs 2.f.i and 2.f.ii
Zoonotic transmission (3): States Parties, with known or suspected zoonotic transmission of mpox, including those where zoonotic transmission is known to occur or has been reported in the past, those where presence of MPXV has been documented in any animal species, and those where infection of animals may be suspected including in domestic pets, livestock or wildlife in newly affected countries. These recommendations apply to all States Parties.
EXTENDED: 3.a. Continue to establish or activate collaborative One Health coordination or other mechanisms at federal, national, subnational and/or local level, as relevant, between public health, veterinary, and wildlife authorities for understanding, monitoring and managing the risk of animal-to-human and human-to-animal transmission in natural habitats, forested and other wild or managed environments, wildlife reserves, domestic and peri-domestic settings, zoos, pet shops, animal shelters and any settings where animals may come into contact with domestic waste.
MODIFIED: 3.b. Continue to undertake detailed case investigations and studies to characterize transmission patterns, including suspected or documented spillovers from, and spillback, to animals. In all settings, and particularly for States Parties in the African and Eastern Mediterranean Regions, case investigation forms should be updated and adapted to elicit information on the full range of possible exposures and modes of both zoonotic and human-to-human transmission. Share the findings of these endeavours including ongoing case reporting with WHO and reporting of animal cases to WOAH. https://www.who.int/publications/i/item/WHO-MPX-Clinical_CRF-2022.3
Development and deployment of medical countermeasures (4): These recommendations applie to all States Parties and particularly including those with capacity to innovate, develop and/or manufacture medical countermeasures
MODIFIED: 4.a. States Parties should continue to pursue and/or support research, development, manufacturing capacity, and where possible technology transfer for mpox diagnostics, vaccines or therapeutics to enhance availability and raise production.
EXTENDED: 4.b. States Parties and manufacturers should continue to work with WHO to ensure diagnostics, vaccines, therapeutics, and other necessary supplies are made available based on public health needs, solidarity and at reasonable cost to countries where they are most needed to support efforts to stop the onward spread of mpox.