December 08, 2023: FDA Clears First Cell-Based Gene Therapies for Sickle Cell Disease
Today, the U.S. Food and Drug Administration sanctioned two groundbreaking treatments, Casgevy and Lyfgenia, marking a historic moment as the initial cell-based gene therapies authorized to address sickle cell disease (SCD) in patients aged 12 and above. Of particular significance, Casgevy stands as the first FDA-approved therapy employing a pioneering genome editing technology, marking a pivotal leap in gene therapy advancements.
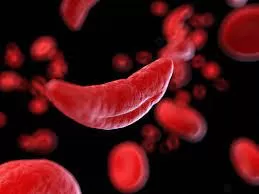
Sickle cell disease, a cluster of inherited blood disorders impacting around 100,000 individuals in the U.S., predominantly affects African and Hispanic Americans. The disease is primarily characterized by a mutation in hemoglobin, the protein responsible for transporting oxygen in red blood cells. This mutation leads to the distinctive sickle or crescent shape of red blood cells, causing vascular obstruction and impeding oxygen delivery to body tissues, resulting in excruciating pain and organ damage termed vaso-occlusive events (VOEs) or crises.
Dr. Nicole Verdun, Director of the Office of Therapeutic Products at the FDA’s Center for Biologics Evaluation and Research, highlighted the significance of these approvals in addressing the substantial unmet needs of individuals severely affected by sickle cell disease. She emphasized the potential of gene therapy to offer more precise and effective treatments, especially for rare diseases with limited therapeutic options.
Casgevy, a cell-based gene therapy, earned approval for treating sickle cell disease in patients aged 12 and above experiencing recurrent vaso-occlusive crises. Notably, it’s the pioneering FDA-approved therapy utilizing CRISPR/Cas9, a genome editing technology. This therapy modifies patients’ hematopoietic (blood) stem cells through CRISPR/Cas9, increasing the production of fetal hemoglobin (HbF), which hinders the sickling of red blood cells.
CRISPR/Cas9 precisely alters DNA, enhancing the production of HbF that prevents red blood cells from sickling in patients with sickle cell disease.
Lyfgenia, another cell-based gene therapy, utilizes a lentiviral vector for genetic modification and is approved for patients aged 12 and above with sickle cell disease and a history of vaso-occlusive events. This therapy modifies blood stem cells to produce HbAT87Q, a hemoglobin resembling normal adult hemoglobin, reducing the risk of sickling in red blood cells.
Both treatments involve modifying patients’ blood stem cells and administering them back as a one-time infusion during a hematopoietic stem cell transplant. Patients will undergo myeloablative conditioning (high-dose chemotherapy) before treatment to prepare their bone marrow for modified cell transplantation. Long-term studies will assess the safety and efficacy of Casgevy and Lyfgenia.
Dr. Peter Marks, Director of the FDA’s Center for Biologics Evaluation and Research, emphasized the significance of these therapies in targeting severe conditions and enhancing public health. These approvals follow thorough evaluations of scientific and clinical data, showcasing the FDA’s dedication to facilitating safe and effective treatments for conditions impacting human health significantly.
The efficacy of Casgevy was assessed in an ongoing trial, where a significant majority of patients achieved desired outcomes. Common side effects included low blood cell levels, mouth sores, nausea, and musculoskeletal pain.
Lyfgenia’s effectiveness was based on a multicenter study, with the majority of patients experiencing resolution of vaso-occlusive events. Common side effects comprised mouth sores and low blood cell levels, typical of chemotherapy.
A note of caution was added for Lyfgenia due to the occurrence of blood cancer in some treated patients, necessitating lifelong monitoring for these malignancies.
Both Casgevy and Lyfgenia received several designations, including Priority Review, Orphan Drug, Fast Track, and Regenerative Medicine Advanced Therapy designations. Vertex Pharmaceuticals Inc. and Bluebird Bio Inc. secured FDA approval for Casgevy and Lyfgenia, respectively.