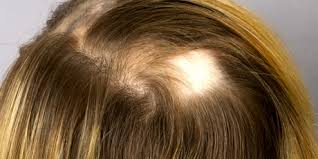
The U.S. Food and Drug Administration (FDA) has recently approved Leqselvi (deuruxolitinib) tablets for the treatment of adults suffering from severe alopecia areata, marking a significant advancement in the management of this challenging condition.
Leqselvi, administered as an 8 mg tablet twice daily, functions as a selective inhibitor of the Janus kinases JAK1 and JAK2. This approval is grounded in robust clinical evidence, particularly the results from the THRIVE-AA1 and THRIVE-AA2 phase 3 clinical trials. These randomized, double-blind, placebo-controlled studies enrolled a total of 1,220 patients who had experienced at least 50 percent scalp hair loss for over six months.
The trials demonstrated promising outcomes, with more than 30 percent of patients taking Leqselvi achieving at least 80 percent scalp hair coverage (Severity of Alopecia Tool [SALT] score ≤20) within 24 weeks. Furthermore, up to one-quarter of the patients experienced nearly complete scalp hair regrowth, attaining 90 percent or greater coverage. Notably, the number of patients achieving a SALT score of ≤20 did not plateau through the 24-week period, indicating sustained improvement over time.
Dr. Natasha Mesinkovska, M.D., Ph.D., from the University of California, Irvine, and an investigator in the Leqselvi clinical development program, emphasized the importance of this treatment: “For many people with severe alopecia areata, early intervention with effective treatment is critical. An oral JAK that delivers proven results will be impactful for the alopecia areata community.”
The safety profile of Leqselvi was also assessed during the trials, with the most common adverse events reported being headache, acne, and nasopharyngitis. Despite these side effects, the benefits observed in hair regrowth make Leqselvi a valuable option for patients with severe alopecia areata.
Sun Pharma, the company behind Leqselvi, received the FDA approval, paving the way for its availability to patients in need.
For further information on the approval and detailed clinical data, visit www.multivu.com/players/uk/928.