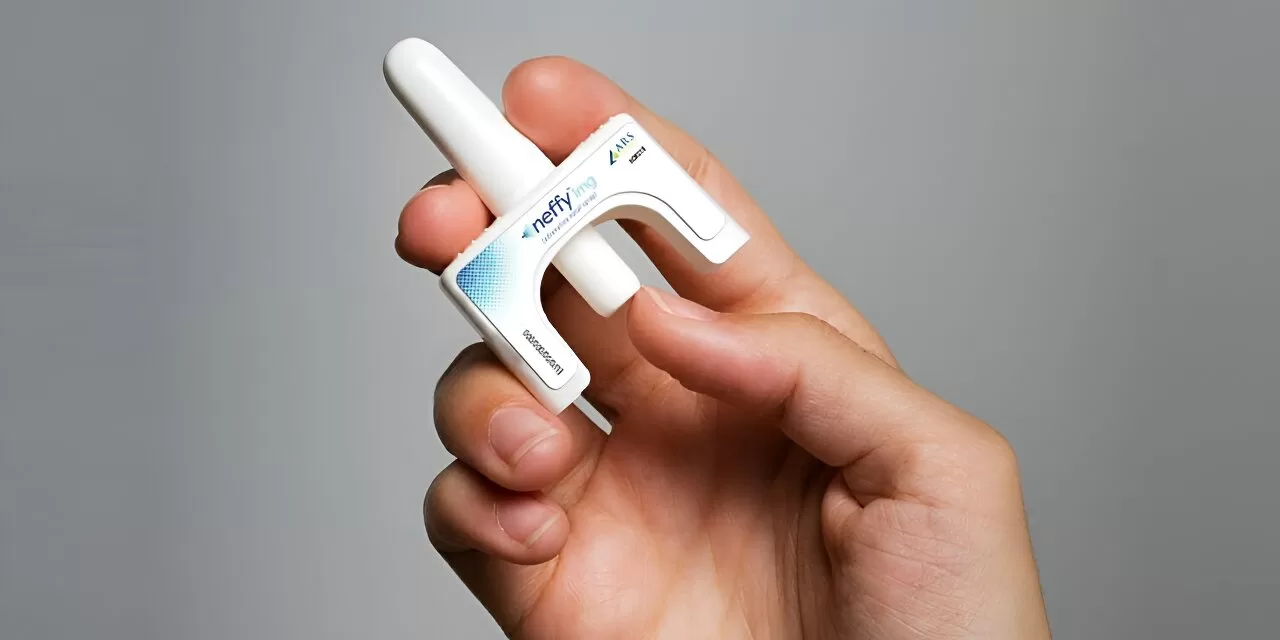
In a groundbreaking development for allergy treatment, the U.S. Food and Drug Administration (FDA) has approved Neffy, the first nasal spray designed to address life-threatening allergic reactions without the need for injections. This new epinephrine nasal spray is set to provide a crucial alternative for those who face difficulties with injectable treatments.
Announced on August 9, 2024, the FDA’s approval marks a significant advancement in the management of anaphylaxis, a severe and potentially fatal allergic reaction triggered by allergens such as certain foods, medications, or insect stings. Neffy is intended for use by adults and children who weigh more than 66 pounds.
Dr. Kelly Stone, Associate Director of the Division of Pulmonology, Allergy, and Critical Care in the FDA’s Center for Drug Evaluation and Research, emphasized the importance of this new treatment option. “Anaphylaxis is life-threatening, and some individuals, particularly children, may delay or avoid treatment due to fear of injections,” Dr. Stone stated. “The availability of epinephrine nasal spray may reduce barriers to rapid treatment of anaphylaxis. As a result, Neffy provides an important treatment option and addresses an unmet need.”
The Neffy nasal spray, developed by ARS Pharmaceuticals, is administered by spraying into one nostril. If the initial dose does not alleviate symptoms, a second dose from a new dispenser may be used in the same nostril. The FDA advises monitoring patients closely after administration to ensure adequate response and to seek emergency medical help if necessary.
The approval of Neffy followed extensive studies involving 175 healthy adults. These studies compared the epinephrine concentrations in the blood after administering Neffy versus approved injectable epinephrine products. The findings revealed that Neffy achieved comparable epinephrine levels and was effective in eliciting the necessary increases in blood pressure and heart rate for treating anaphylaxis.
While Neffy offers a promising new option, it may come with side effects including throat irritation, nasal discomfort, headache, dizziness, nausea, and more. The FDA has issued warnings for individuals with certain nasal conditions, such as nasal polyps or a history of nasal surgery, as these may impact the absorption of Neffy. Patients with these conditions are advised to consult with a healthcare professional regarding the use of injectable epinephrine.
The approval follows an expert advisory panel’s endorsement of Neffy in May 2023 and a subsequent request for additional studies before final approval in September 2023.
For more information on recognizing and managing anaphylaxis, visit the Cleveland Clinic’s resources.
Source: U.S. Food and Drug Administration, announcement, August 9, 2024