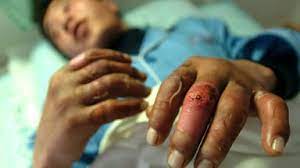
The U.S. Food and Drug Administration (FDA) has granted approval for Aurlumyn (iloprost) injection as the first-ever treatment option for severe frostbite in adults, aimed at reducing the risk of finger or toe amputation, announced Norman Stockbridge, M.D., Ph.D., director of the Division of Cardiology and Nephrology in the FDA’s Center for Drug Evaluation and Research.
Severe frostbite, a condition where both the skin and underlying tissue freeze, can lead to the cessation of blood flow and, in severe cases, necessitate amputation. Aurlumyn, containing iloprost as its active ingredient, functions as a vasodilator, aiding in the opening of blood vessels, and prevents blood clotting.
The efficacy of iloprost in treating severe frostbite was demonstrated in a controlled trial involving 47 adults with severe frostbite. The patients were randomized into three treatment groups, one of which received iloprost intravenously for up to 8 days. The primary measure of efficacy was based on a bone scan conducted 7 days after initial frostbite to predict the need for amputation of at least one finger or toe.
Results showed that on day 7, none of the patients receiving iloprost alone exhibited bone scan abnormalities predictive of amputation, whereas 19% and 60% of patients in the other treatment groups did. The incidence of bone scan abnormalities was significantly lower in the iloprost-treated groups, consistent with subsequent amputation outcomes.
Common side effects of Aurlumyn include headache, flushing, heart palpitations, nausea, vomiting, dizziness, and low blood pressure. It carries a warning for symptomatic hypotension. Aurlumyn received Priority Review and Orphan Drug designations for this indication.
Iloprost, the active ingredient in Aurlumyn, was initially approved in 2004 for the treatment of pulmonary arterial hypertension.
The approval of Aurlumyn marks a significant milestone in severe frostbite treatment, offering physicians a vital tool to prevent the life-altering amputation of frostbitten fingers or toes. The FDA granted approval of Aurlumyn to Eicos Sciences Inc., paving the way for improved outcomes and enhanced patient care in severe frostbite cases.