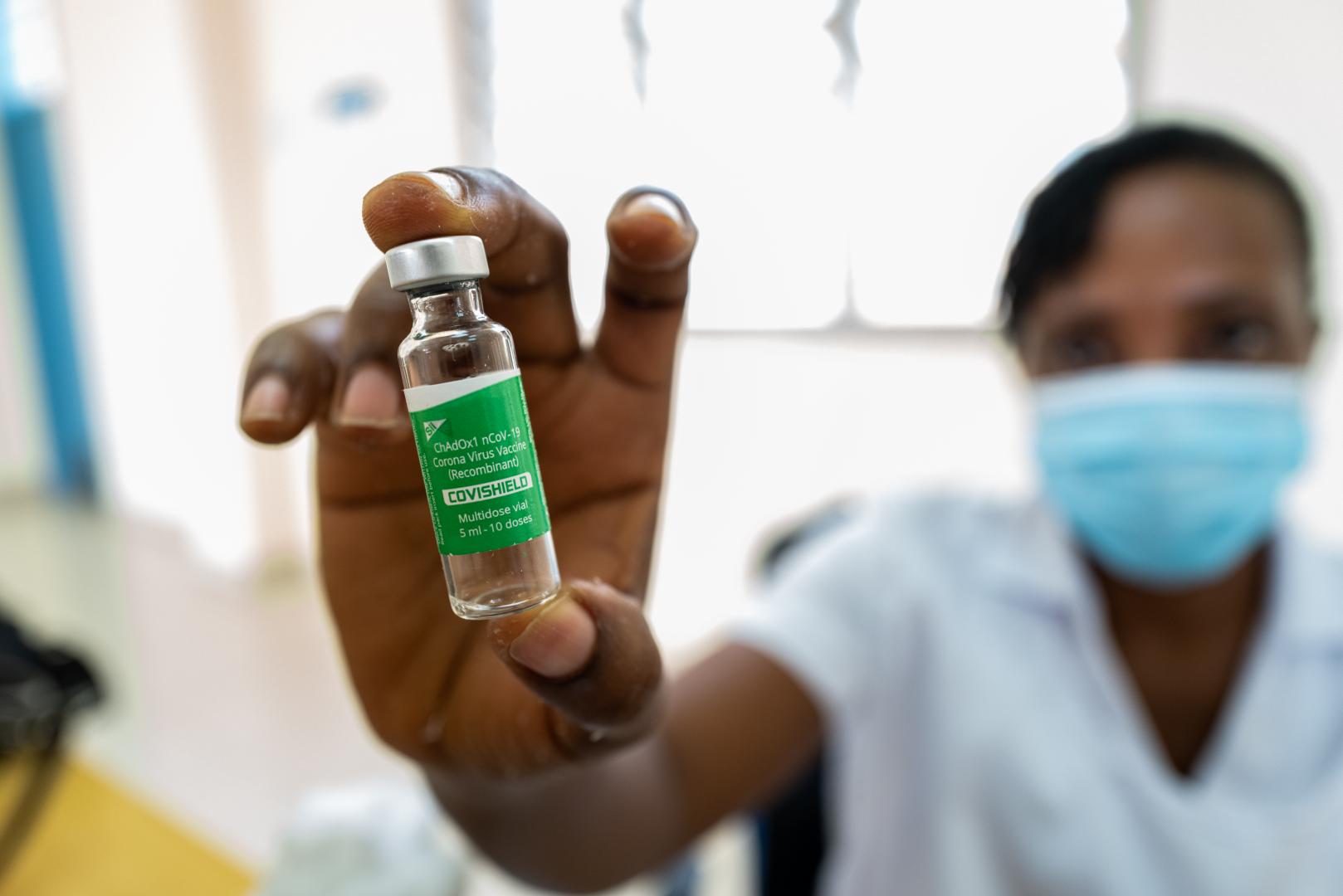
Brazzaville – The WHO Regional Office for Africa wishes to provide clarification and guidance on the issue of expiry dates of COVID-19 vaccines in general, and specifically on AstraZeneca vaccine doses produced by the Serum Institute of India (SII) – referred to as Covishield, that were delivered to several African countries and have since expired.
Vaccines, like all other medicinal products, come with a date of expiry and shelf-life determined by the manufacturer and approved by regulatory authorities. Given the urgency of the COVID-19 pandemic, initial emergency use authorization on a number of COVID-19 vaccines, including the AstraZeneca Covishield vaccine was given by regulators when only six months worth of data for assignment of the expiration date was available.
For any extension to the shelf-life of COVID-19 vaccines that have received WHO Emergency Use Listing (EUL), the data from manufacturers will be reviewed and the outcomes disseminated to all country’s national regulatory authorities for their decision making.
National regulatory authorities oversee all post approval changes to COVID-19 vaccines including any changes to their shelf-life and expiry dates in their countries. Since reliance on EUL has been a crucial factor in national authorization of COVID-19 vaccines, national regulatory authorities can make decisions based on the EUL assessment of stability data from the vaccine manufacturer and recommended shelf-life.
Shelf life and expiry of Covishield vaccines
- In March, the African Union’s African Vaccine Acquisition Task Team (AVATT) redistributed 925 000 doses of Covishield vaccine produced by SII to 13 African countries with an expiry date of 13 April 2021. The bulk of these vaccine doses were administered, but some countries have unused doses remaining.
- Any vaccine that has passed its expiry date, including Covishield, should not be administered. While discarding vaccines is deeply regrettable in the context of any immunization programme, WHO recommends that these expired doses should be removed from the distribution chain and safely disposed.
- The shelf-life of a vaccine is a reflection of how long the vaccine retains its potency and stability at a given storage temperature and therefore its effectiveness. The shelf-life is used to establish the expiry date of each batch of the vaccine product. Expiry dates do not affect the safety of the vaccine, rather are related to the potency or amount of protection the vaccine gives.
- The WHO prequalification team is expecting to receive additional stability data for Covishield from SII. This data will enable WHO to do a rigorous review and approve any proposed extension to the vaccine shelf-life.
- Any extension in the shelf-life will only apply to vaccines not yet labelled and distributed. Therefore, the expired or near to expire doses in distribution for use will not be affected by the future decision of shelf-life extension. This decision is expected to be communicated to all countries’ national regulatory authorities in due course.
Safe disposal of expired COVID-19 vaccines
- There is no special procedure applied for disposing COVID-19 vaccines. Disposal should follow the existing national guidelines for the safe disposal of vaccines. If a standard protocol does not exist, countries can refer to the ‘WHO Guidelines for the Safe Disposal of Unwanted Pharmaceuticals In and After an Emergency’ which can be accessed here.
- The WHO Regional Office for Africa’s ‘Standard Operational Procedures for Waste Management to Ensure the Safe Disposal of COVID-19 vaccines’ can be accessed here.
- Immunization programmes should support health workers with information about the verification and disposal of expired doses. Broader public communications may also be required to reassure that expiry dates issues are more about vaccine potency and performance and are not a safety issue.
Tracking expiry dates
- Synchronizing vaccination campaigns with the shelf-life of a vaccine at the time of its arrival in a country is key to facilitating consumption of the supply before they expire. Countries should continue to track and monitor expiry dates at regular intervals.
- WHO and COVAX partners have provided support to countries to ensure that national deployment and vaccination plans (NDVPs) include data driven planning and close monitoring of expiry dates across the supply chain to prevent and minimise wastage.
- Further details of the ‘Effective Vaccine Management’ initiative can be found here.
- WHO and UNICEF have developed the ‘COVID-19 Vaccination: Supply and Logistics guidance’ which can be accessed here.
- WHO will continue to provide updates on vaccine expiry dates and their shelf-life as further data becomes available.