January 25, 2025 – The Lancet Digital Health
A recent viewpoint paper published in The Lancet Digital Health explores the remarkable progress and significant challenges in the research and development of brain implants. Led by Stanisa Raspopovic from MedUni Vienna, the paper calls for heightened ethical and scientific attention as clinical trials move from animal models to human testing.
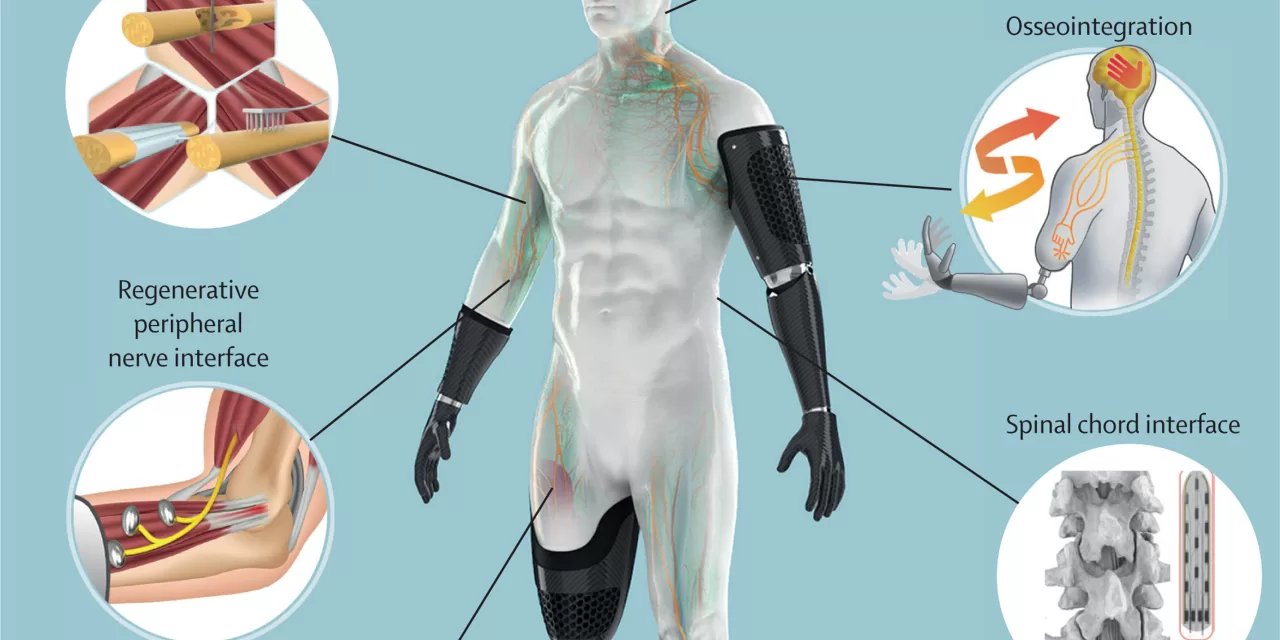
The innovative technology behind brain implants has recently attracted global attention, offering renewed hope for individuals suffering from neurological disorders. Neural implants have demonstrated potential benefits, ranging from restoring mobility to alleviating pain and improving sensory functions. However, researchers are stressing the need for careful ethical consideration due to the profound psychological effects these implants can have on patients.
One of the most striking examples of progress in this field comes from a clinical trial in the United States, where a paraplegic patient was implanted with a brain chip. The device allowed the man to control his wheelchair, operate a computer, and even play chess. But as the months progressed, the patient experienced a decrease in the accuracy of the cursor control, and a delay between his thoughts and the computer’s actions.
“The issue highlights one of the many challenges faced in this technology’s development,” said Stanisa Raspopovic, who co-authored the paper with Marcello Ienca (Technical University of Munich) and Giacomo Valle (ETH Zurich). The team emphasized the importance of addressing logistical questions, such as who will handle device maintenance post-study and whether patients will still have access to the device after a clinical trial concludes.
Beyond the technical hurdles, the study emphasizes the psychological implications of neural implants. These devices, which interface directly with the nervous system, could potentially influence cognitive processes, emotions, and even free will. As a result, researchers assert that traditional methods for assessing safety and efficacy, often used in drug trials, are inadequate for studying such complex systems. New models are required to ensure the subjective experiences of patients are accurately evaluated, while also safeguarding their psychological privacy.
In addition to psychological concerns, the technological aspects of neuroprostheses raise critical issues of data security. Neural implants collect and process sensitive neuronal data, which is far more vulnerable than other forms of medical data. The potential for data breaches and cyber-attacks means that the implementation of rigorous security protocols is essential.
“The use of neural implants cannot be reduced to just medical risks,” Raspopovic explained. “We are in the early stages of clinical trials, and we must address these ethical and technical questions now, not only when problems arise with test subjects or patients.”
Disclaimer: The information provided in this article is based on a recent study published in The Lancet Digital Health and is for informational purposes only. It does not constitute medical advice. Please consult healthcare professionals for any medical concerns or questions regarding neural implants and related treatments.
For further reading, please see the original study by Marcello Ienca et al, Clinical Trials for Implantable Neural Prostheses: Understanding the Ethical and Technical Requirements, The Lancet Digital Health (2025). DOI: 10.1016/S2589-7500(24)00222-X.