The European Medicines Agency (EMA) has expanded its reach beyond the European Union (EU) by recommending two crucial drugs for combating neglected tropical diseases—schistosomiasis and sleeping sickness. Through a special procedure called EU Medicines for All (EU-M4All), conducted in collaboration with the World Health Organization (WHO), the EMA offers scientific evaluations for essential medications intended for markets outside the EU.
One of the recommended drugs, Arpraziquantel, targets schistosomiasis, a debilitating condition caused by blood flukes that can severely damage organs like the bladder, kidneys, and liver. It’s a prevalent disease across Africa and in parts of South America, the Caribbean, the Middle East, and Asia, affecting over 250 million individuals annually and causing at least 20,000 deaths. The newly recommended formulation of Arpraziquantel specifically caters to children aged 3 months to 6 years, addressing a critical gap in pediatric treatment.
Arpraziquantel’s efficacy in treating schistosomiasis has been supported by extensive clinical trials, showcasing cure rates of 90% or higher. Its development was spurred by the Pediatric Praziquantel Consortium, funded by the European and Developing Countries Clinical Trials Partnership (EDCTP) and the Global Health Innovative Technology Fund in Japan.
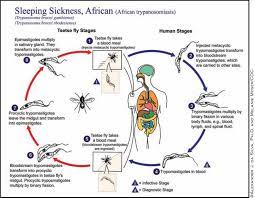
The other recommended drug, Fexinidazole, tackles human African trypanosomiasis, commonly known as sleeping sickness, caused by Trypanosoma brucei rhodesiense transmitted via tsetse flies in Africa. This oral treatment, designed for both adults and children aged 6 years or older and weighing at least 20 kg (44 lb), proved effective in clinical trials conducted in Malawi and Uganda by the Drugs for Neglected Diseases Initiative (DNDi), supported by the EDCTP.
The EU-M4All initiative, introduced in 2004, initially aimed at extending the reach of HIV/AIDS treatments. Over time, it has evolved to cover a broader spectrum of products and therapeutic areas. Notably, the scheme’s impact has been substantial, resulting in 15 positive opinions and 158 regulatory approvals in 93 countries by the latest count. This widened scope has significantly enhanced global accessibility to vital medications.
In recent years, the EU-M4All process has undergone further enhancements, now allowing parallel execution with a centralized procedure to expedite global medicine accessibility. The criteria for eligibility have also broadened, encompassing various product types and therapeutic areas, provided they gain approval from both the EMA and the WHO. These advancements underscore the initiative’s commitment to improving healthcare delivery and patient outcomes on a global scale.