Research reveals that targeting protein IGFBP7 could improve insulin secretion, offering new hope for Type 2 Diabetes treatment.
In a groundbreaking study led by researchers at Lund University, a protein known as IGFBP7 has been shown to play a significant role in impairing the pancreas’s ability to release insulin in individuals with type 2 diabetes. This discovery opens the door for new therapeutic approaches that could improve insulin secretion and help combat the chronic disease that affects millions worldwide.
Type 2 diabetes is characterized by the body’s inability to produce sufficient insulin, a hormone essential for regulating blood glucose levels. Over time, the condition leads to elevated blood sugar and increases the risk of severe complications, including cardiovascular disease and nerve damage. Despite extensive research, the mechanisms behind the impaired insulin secretion in type 2 diabetes have remained elusive—until now.
A team of researchers, led by Professor Lena Eliasson at Lund University and in collaboration with Newcastle University, focused on understanding how beta cells, located in the islets of Langerhans in the pancreas, regulate insulin secretion. Their work has shed light on the role of IGFBP7, a protein secreted from the liver that has been studied for its links to heart failure and kidney damage.
What makes IGFBP7 particularly interesting is that, while its role in insulin secretion was not previously understood, the new research suggests it may have a substantial impact on beta cell function in people with type 2 diabetes.
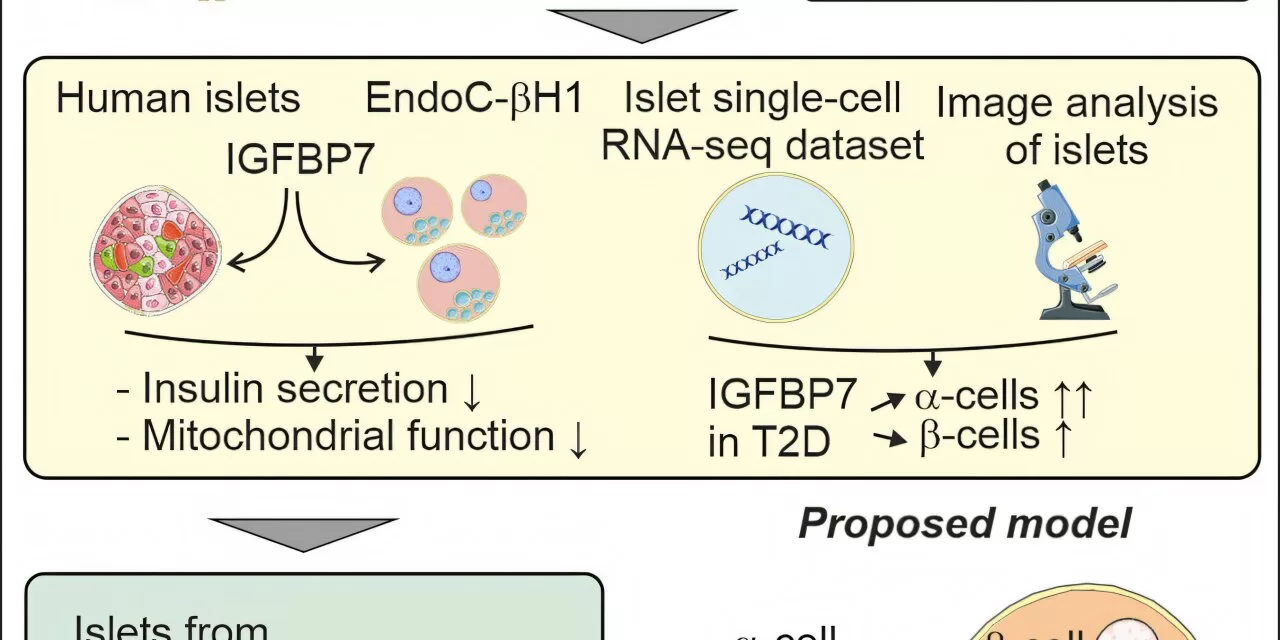
The research team discovered that IGFBP7 levels were elevated in the beta cells of individuals with type 2 diabetes. In a series of experiments, the team treated beta cells from non-diabetic individuals with IGFBP7 and observed a notable reduction in insulin secretion compared to a control group. Furthermore, they found that the treated cells had lower expression of another protein essential for healthy cell function, indicating that IGFBP7 impairs beta cell performance.
Professor Eliasson, who has dedicated years to researching the mechanisms of insulin secretion, remarked, “This suggests that elevated levels of IGFBP7 impair the function of beta cells and their ability to secrete insulin. Our findings provide a crucial piece of the puzzle in understanding why insulin secretion is diminished in type 2 diabetes.”
To further investigate this link, the researchers knocked out the IGFBP7 gene in beta cells from individuals with type 2 diabetes. The result was striking: insulin secretion increased. This promising outcome suggests that reducing the levels or expression of IGFBP7 could offer a novel approach to improving insulin release in diabetic patients.
Efraim Westholm, the first author of the study and a recent PhD graduate from Lund University, commented, “We believe IGFBP7 could be a drug target for type 2 diabetes. If we can develop treatments that reduce its expression, we may improve insulin secretion and offer new hope to people living with the disease.”
The researchers caution that further studies are needed, including trials involving cells from more participants, to deepen the understanding of how IGFBP7 behaves in different individuals and to confirm its role in type 2 diabetes. Nevertheless, these initial findings bring exciting possibilities for future therapies that could address not only insulin secretion but potentially the broader impact of IGFBP7 on other organs such as the heart, liver, and kidneys.
For now, Eliasson and her team are hopeful that their work can pave the way for the development of targeted treatments that may improve the quality of life for those with type 2 diabetes. As Westholm adds, “It is crucial to see the big picture when it comes to type 2 diabetes, and we believe IGFBP7 could play an important role in improving organ function.”
The study, titled “IGFBP7 is upregulated in islets from T2D donors and reduces insulin secretion,” was published in the journal iScience.
Source: Efraim Westholm et al, iScience (2024). DOI: 10.1016/j.isci.2024.110767