In the ongoing battle against influenza viruses, Duke University researchers have achieved a significant breakthrough by devising a vaccine that prompts the immune system to target a less variable portion of the virus surface. This promising approach, demonstrated through successful experiments with mice and ferrets, could pave the way for more broadly protective influenza vaccines, potentially reducing the need for annual shots tailored to specific strains.
Published on May 1 in the prestigious journal Science Translational Medicine, this novel vaccine strategy marks a milestone in a five-year-long endeavor to develop a longer-lasting universal flu vaccine capable of thwarting all variants of the virus.
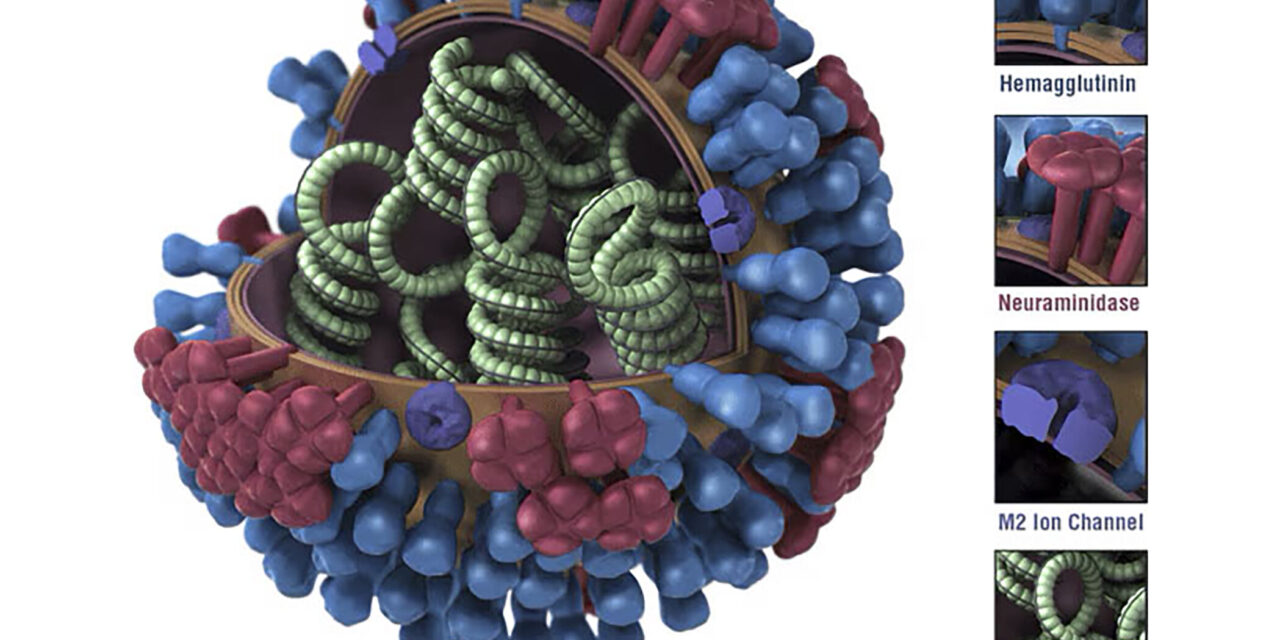
Nicholas Heaton, Ph.D., an associate professor of molecular genetics and microbiology at Duke, spearheaded the research. He explains the significance of the approach, stating, “The strongest correlates of protection have to do with hemagglutinin-directed immunity.” Traditionally, flu vaccines target the bulb-like “head” of the hemagglutinin protein, which undergoes frequent changes, necessitating annual vaccine updates. In contrast, the stalk region of hemagglutinin exhibits far less variability, offering a more stable target for vaccine design.
To capitalize on this stability, the Duke team employed gene-editing techniques to create over 80,000 variants of the hemagglutinin protein, focusing on alterations in the head domain. By exposing the immune system to a diverse array of head conformations while maintaining the consistency of the stalk region, the experimental vaccine prompted a robust antibody response directed at the stalk portion. This dual response, targeting both head and stalk regions, holds immense promise for comprehensive protection against diverse influenza strains.
Heaton elucidates the implications of their findings, stating, “Antibodies against the stalk work differently… Their mechanism of protection is not necessarily to block the first step of infection.” Consequently, the experimental vaccine not only conferred immunity to the tested influenza strains but also demonstrated potential efficacy against unforeseen variants or pandemics.
In laboratory tests, the highly variant vaccine conferred remarkable protection, with 100% of mice avoiding illness or death following exposure to a lethal dose of flu viruses.
Moving forward, the researchers aim to streamline their approach by identifying whether presenting fewer than 80,000 hemagglutinin variants can achieve comparable levels of immunity. This ongoing research holds promise for revolutionizing flu vaccination strategies, potentially saving countless lives worldwide.
In the perpetual battle against influenza, Duke University researchers have unleashed a formidable weapon—one that promises to tip the scales in favor of broader protection and reduced reliance on seasonal flu shots.