Researchers at Baylor College of Medicine and MD Anderson Cancer Center identify key structures involved in norovirus replication, opening the door for potential antiviral therapies.
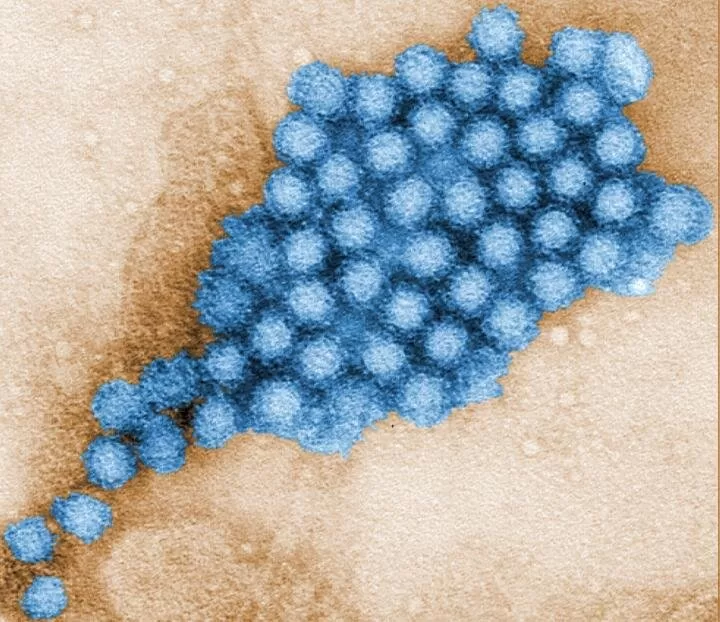
Human norovirus, a leading cause of viral gastroenteritis worldwide, affects an estimated 685 million people annually and is responsible for around 212,000 deaths each year. Despite its widespread impact, there are currently no approved vaccines or antiviral treatments to combat this highly infectious virus. However, a breakthrough discovery from researchers at Baylor College of Medicine and the University of Texas MD Anderson Cancer Center, published in Science Advances, could pave the way for new therapeutic options.
The team has identified specialized compartments within infected cells, known as replication hubs, where the virus replicates. These findings could ultimately lead to the development of antiviral drugs that target these viral factories and help prevent, control, or treat norovirus infections.
“When viruses infect cells, they typically create specialized compartments—replication factories—where new virus particles are produced,” explained Dr. Soni Kaundal, first author of the study and postdoctoral associate in the Verna and Marrs McLean Department of Biochemistry and Molecular Pharmacology at Baylor. “However, little was known about the replication factories of norovirus, making this discovery a significant step forward.”
Recent research has shown that many viral replication factories are not enclosed by membranes, as one might expect. Instead, they are biomolecular condensates—liquid-like structures formed by a process known as liquid-liquid phase separation. These condensates selectively incorporate proteins and other necessary materials for viral replication. While such condensates have been identified in other viruses, such as rabies and measles, their role in norovirus had not been explored until now.
The research team began by analyzing norovirus proteins to determine which ones might form liquid-like condensates. The results indicated that the RNA-dependent RNA polymerase (RdRp) of the predominant human norovirus strain, GII.4, had the highest potential to form these condensates. This protein, which binds to the virus’s genetic material and plays a central role in viral replication, possesses all the characteristics required for condensate formation, including a flexible region and the ability to form oligomers.
Experimental tests confirmed that GII.4 RdRp does, indeed, form dynamic liquid condensates in laboratory conditions. These condensates behave like liquid droplets, merging and dividing while exchanging materials with their surroundings. According to Dr. B.V. Venkataram Prasad, corresponding author of the study, these findings demonstrate that the viral RNA polymerase drives the formation of condensates that play a crucial role in viral replication.
“We have shown that these liquid-like condensates are not only formed in lab-grown human cells but also in human intestinal enteroid cultures—miniature models of the human gastrointestinal tract that simulate real infection conditions,” Prasad said. “This reinforces the idea that these condensates are central to norovirus replication.”
Norovirus has long been difficult to study in laboratory settings, but advances in cultivating human norovirus strains in human intestinal enteroids have made it possible to study the virus’s replication process in more detail. This breakthrough has enabled the researchers to examine how the virus forms replication hubs within infected cells, a crucial step in understanding how the virus hijacks the host’s machinery to replicate.
The discovery of liquid-like replication hubs has important implications for the design of antiviral therapies. Since these condensates are central to viral replication, targeting them could provide a novel approach to blocking norovirus infection. The research team’s bioinformatic analysis suggests that the ability to form such replication factories may be a common feature among most norovirus strains, further emphasizing the potential for a broad-spectrum antiviral strategy.
Dr. Mary Estes, a key contributor to the study and a pioneer in norovirus research, expressed her excitement about the findings. “This is a remarkable paper that provides valuable insights into how norovirus replicates inside the human body,” Estes said. “It validates the findings in virus-infected cells and underscores the importance of using human intestinal enteroids for studying norovirus infection.”
The study’s findings also shed light on the challenges that norovirus poses, particularly for vulnerable populations, such as children and immunocompromised patients. By uncovering the mechanisms behind norovirus replication, the researchers hope to contribute to the development of antiviral treatments that could alleviate the global burden of this infectious disease.
This research was supported by several contributors, including Ramakrishnan Anish, B. Vijayalakshmi Ayyar, Sreejesh Shanker, Gundeep Kaur, Sue E. Crawford, Jeroen Pollet, and Fabio Stossi.
For more information, refer to the original publication: Kaundal, S., et al. “RNA-dependent RNA polymerase of predominant human norovirus forms liquid-liquid phase condensates as viral replication factories.” Science Advances (2024). DOI: 10.1126/sciadv.adp9333.