University of California, Irvine Researchers Unveil Key Insights into RNA Processing Defects Linked to Huntington’s Disease
A groundbreaking study led by researchers at the University of California, Irvine (UCI) has unveiled critical molecular mechanisms underlying Huntington’s disease (HD), offering promising new therapeutic targets. This discovery, published in Nature Neuroscience, not only sheds light on the complex nature of HD but also suggests potential therapeutic strategies that could extend to other neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), frontotemporal lobar dementia (FTLD), and Alzheimer’s disease.
While it has long been known that HD is caused by the abnormal expansion of cytosine, adenine, and guanine nucleotide repeats in the gene responsible for the disease, the precise molecular processes through which this mutation disrupts cellular functions have remained elusive. This study has made a breakthrough by revealing how disruptions in RNA processing, critical to gene regulation, play a central role in HD pathology.
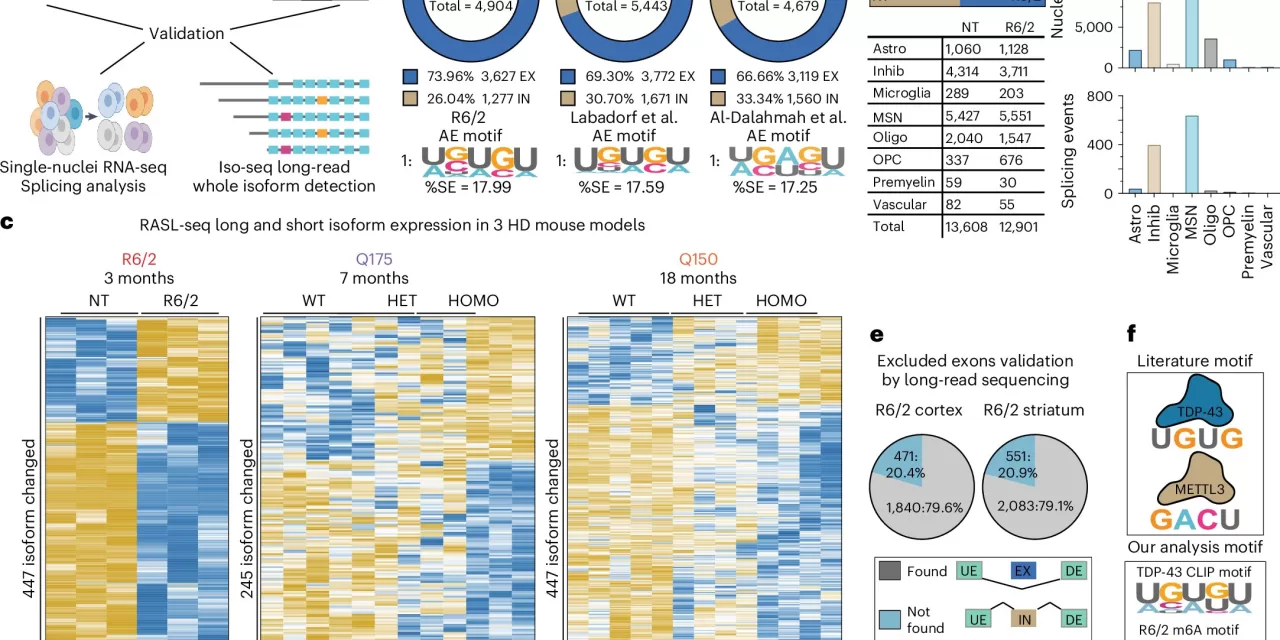
The research identifies the interplay between two important RNA processing regulators: the RNA-binding protein TDP-43 and the m6A RNA modification chemical tag. In the study, both TDP-43 and m6A modification patterns were found to be altered in genes that are dysregulated in HD, highlighting their role in defective RNA processing. This finding is significant, as TDP-43 is traditionally associated with ALS and FTLD, yet the same pathology was observed in the brains of HD patients, suggesting a shared molecular disruption across these diseases.
Dr. Leslie Thompson, co-corresponding author and UC Irvine Chancellor’s Professor of Psychiatry & Human Behavior and Neurobiology & Behavior, emphasized the implications of these findings: “Our discoveries offer new insights into the role of TDP-43 and m6A modifications in defective RNA processing in HD. This enhanced understanding of their involvement opens up the possibility of developing drugs that target these pathways, potentially slowing or even reversing neurodegeneration in HD, ALS, and other diseases where TDP-43 dysregulation is significant.”
The study, spearheaded by UC Irvine assistant project scientist Thai B. Nguyen, used advanced genomic and molecular biology techniques to explore how m6A RNA modifications act as landmarks that direct TDP-43 to regulate critical RNA molecules. The research utilized invaluable tissue samples from global brain banks to investigate the complex process of RNA splicing—a vital component of gene expression that is disrupted in neurodegenerative conditions like HD.
The team discovered that both HD mouse models and human patients exhibited mislocalization of TDP-43 and alterations in m6A RNA modifications. These disruptions impeded TDP-43’s ability to bind to RNA accurately, leading to abnormal RNA processing and splicing errors. These molecular irregularities were found to correspond with gene disruptions, particularly in the striatum, a brain region heavily affected by HD-related neuronal dysfunction.
Dr. Robert Spitale, co-corresponding author and UC Irvine professor of Pharmaceutical Sciences, highlighted the significance of this collaboration in advancing research on RNA modifications: “By targeting key processes like RNA splicing and modification, we’re not only advancing our understanding of HD’s molecular disruptions but also paving the way for potential treatments for a broader spectrum of neurodegenerative diseases. This research is the result of a powerful collaboration that combined chemical and genomic tools to uncover this novel mechanism.”
The UCI team partnered with leading experts, including Clotilde Lagier-Tourenne, associate professor of neurology at Harvard University, and Don Cleveland, chair of cellular and molecular medicine at UC San Diego. Additional contributions came from researchers at UC Irvine, Columbia University, the Massachusetts Institute of Technology, the University of Auckland, and Ionis Pharmaceuticals in Carlsbad.
This research represents a significant step forward in the fight against Huntington’s disease, offering hope for new treatments targeting RNA processing mechanisms that could benefit patients suffering from HD and related neurodegenerative disorders.
For further reading, see the full study: Thai B. Nguyen et al., Aberrant Splicing in Huntington’s Disease Accompanies Disrupted TDP-43 Activity and Altered m6A RNA Modification, Nature Neuroscience (2025). DOI: 10.1038/s41593-024-01850-w.