Ithaca, NY—In a groundbreaking discovery, researchers from Weill Cornell Medicine and the Boyce Thompson Institute at Cornell University have uncovered a new pathway through which the gut microbiota and the body work together to regulate fat metabolism and cholesterol levels. The findings, published in the prestigious journal Nature, could pave the way for novel treatments for metabolic disorders and obesity-related conditions.
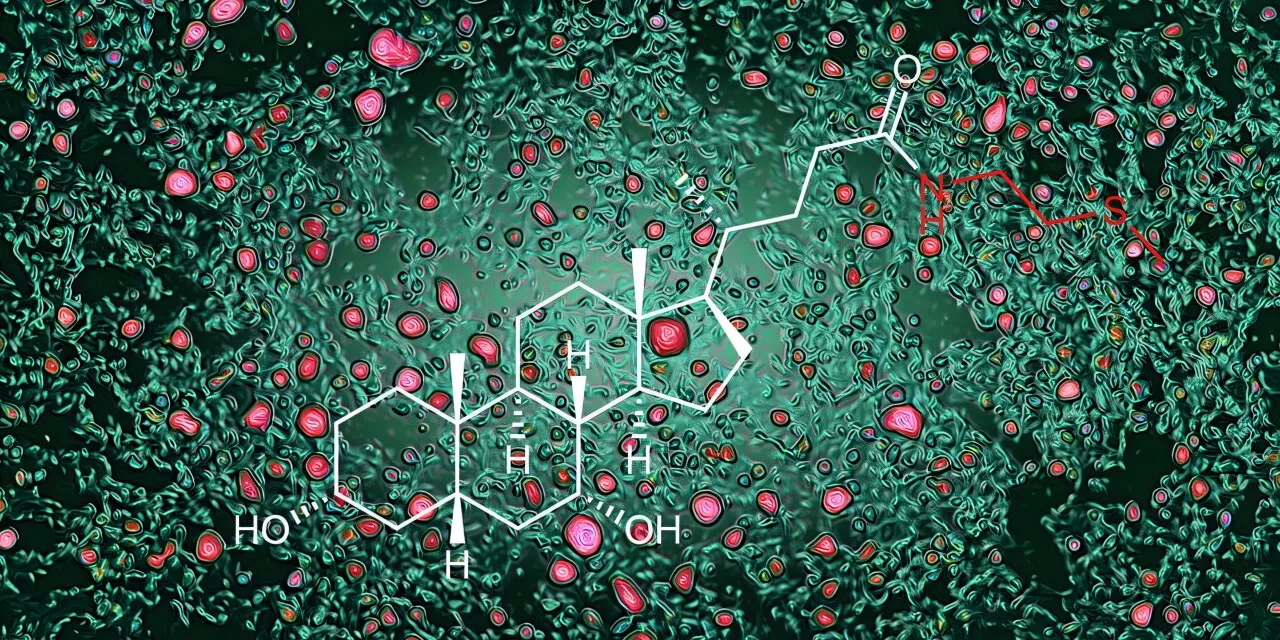
The study highlights the intricate relationship between the human body and gut microbes, which co-evolved to aid in nutrient absorption and food digestion. Central to this relationship is the production of bile acids—molecules derived from cholesterol in the liver that promote fat digestion in the intestine. However, this research goes further, unveiling how bile acids act as signaling molecules to regulate cholesterol and fat metabolism.
A New Discovery in Bile Acid Regulation
Previous studies showed that gut bacteria transform bile acids into a form that activates the FXR receptor, reducing bile acid production. However, the new study reveals a complementary mechanism: an enzyme produced by intestinal cells converts bile acids into a novel form called bile acid-methylcysteamine (BA-MCY). This molecule inhibits FXR, encouraging bile acid production and enhancing fat metabolism.
“Our study reveals there is a dialogue occurring between the gut microbes and the body that is vital for regulating bile acid production,” said Dr. David Artis, co-corresponding author and director of the Jill Roberts Institute for Research in Inflammatory Bowel Disease at Weill Cornell Medicine.
A Multidisciplinary Collaboration
Led by Dr. Artis and Dr. Frank Schroeder of the Boyce Thompson Institute, the research utilized untargeted metabolomics—a technique to identify all molecules in a sample. By comparing molecules in mice with and without gut microbes, researchers identified BA-MCY as a key player in balancing bile acid production.
“The BA-MCYs demonstrate a new paradigm: molecules that are not produced by gut microbes but are still dependent on their presence,” explained Dr. Tae Hyung Won, a co-first author of the study.
This balancing act is crucial. When gut bacteria produce bile acids that activate FXR strongly, the body counters by producing BA-MCYs to maintain equilibrium.
Promising Implications for Human Health
In preclinical models, boosting BA-MCY levels reduced fat accumulation in the liver and suggested potential therapeutic applications for fatty liver disease, high cholesterol, and obesity-related disorders. Dietary fiber intake also increased BA-MCY production, pointing to diet as a potential intervention.
“Importantly, BA-MCYs were also detected in human blood samples, indicating that a similar mechanism may occur in people,” said Dr. Mohammad Arifuzzaman, another co-lead researcher.
The study underscores the potential of dietary strategies, such as increased fiber consumption, to support metabolic health. Researchers plan to further investigate this microbe-host dialogue in disease contexts, from inflammation to cancer.
A Roadmap for Future Research
“This balancing act between gut microbes and the body may help us understand how the microbiota impacts a wide range of diseases,” said Dr. Artis. “Our paper is a roadmap to using metabolomics and chemistry to uncover these complex interactions.”
This groundbreaking research sets the stage for innovative treatments for metabolic and inflammatory diseases, emphasizing the profound impact of the gut microbiota on overall health.
For more information, see the study: Tae Hyung Won et al, Host metabolism balances microbial regulation of bile acid signalling, Nature (2025). DOI: 10.1038/s41586-024-08379-9.