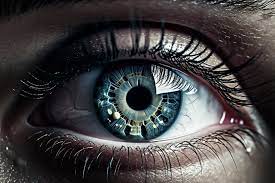
Two major studies from the University of Southern Denmark (SDU) have raised concerns about the diabetes medication Ozempic, revealing a significant increase in the risk of developing a severe eye condition. The studies, conducted by experts in ophthalmology and pharmaceuticals, show that type 2 diabetes patients using Ozempic face a higher risk of optic nerve damage, potentially leading to permanent vision loss.
Ozempic, widely prescribed for managing type 2 diabetes, has been under scrutiny following earlier reports of its link to non-arteritic anterior ischemic optic neuropathy (NAION), a rare but serious condition that damages the optic nerve due to disrupted blood flow. This latest research, based on extensive Danish health registries, is among the largest of its kind in the world and provides further confirmation of the drug’s potential risks.
Jakob Grauslund, Professor of Ophthalmology, led one of the studies, analyzing data from over 400,000 Danes with type 2 diabetes. His findings indicate that Ozempic more than doubles the risk of developing NAION, a condition that can lead to severe and permanent vision loss. Grauslund’s study also highlights a disturbing trend in Denmark, where the incidence of NAION has risen sharply since Ozempic became available in 2018, with up to 150 cases now reported annually, compared to previous years when the number was between 60 and 70.
“We found that the risk is doubled,” Grauslund explained. “Since Ozempic’s introduction, we’ve noticed a significant rise in NAION cases, especially among type 2 diabetes patients.” The study controlled for various factors, including age, gender, and blood sugar levels, to ensure the reliability of the results.
A separate study by Anton Pottegård, Professor of Pharmaceuticals, confirmed Grauslund’s findings using a different methodology. Pottegård and his team examined a smaller, more specific sample of type 2 diabetes patients, comparing new users of Ozempic with patients using other diabetes medications. This study, conducted in collaboration with the Norwegian Institute of Public Health, also found that Ozempic doubled the risk of NAION.
Despite the serious implications of these findings, both researchers emphasized that NAION remains a rare side effect of Ozempic. “It’s important to understand that this is a very rare side effect, and the absolute risk for most patients is still low,” Pottegård stated. “However, these results are crucial for informed discussions between doctors and patients about the benefits and risks of Ozempic treatment.”
The studies also offer insights into the broader context of type 2 diabetes treatment. Kurt Højlund, a professor of diabetes at Steno Diabetes Center Odense, noted that while Ozempic is effective in managing blood sugar, alternative treatments may be preferable for patients who are particularly concerned about the risk of vision loss. New medications protecting against kidney and cardiovascular disease are becoming increasingly popular as options for type 2 diabetes management.
Although the risk of NAION is low, Højlund advises stopping Ozempic treatment if NAION is detected in one eye, as continuing the drug could worsen the condition.
The findings have been shared with Danish and international health authorities, and will likely influence ongoing discussions about the safety of Ozempic. NAION as a potential side effect of semaglutide, the active ingredient in Ozempic, has been under review by regulators for several months.
The two studies were published in the International Journal of Retina and Vitreous and the medRxiv preprint server. They provide critical new data on the risks associated with Ozempic, reinforcing the importance of monitoring for rare side effects in patients receiving the medication.
For more information on the studies, visit the International Journal of Retina and Vitreous and medRxiv.
References:
- Grauslund et al, “Once-weekly semaglutide doubles the five-year risk of nonarteritic anterior ischemic optic neuropathy in a Danish cohort of 424,152 persons with type 2 diabetes,” International Journal of Retina and Vitreous.
- Emma Simonsen et al, “Use of semaglutide and risk of non-arteritic anterior ischemic optic neuropathy: A Danish–Norwegian cohort study,” medRxiv (2024).