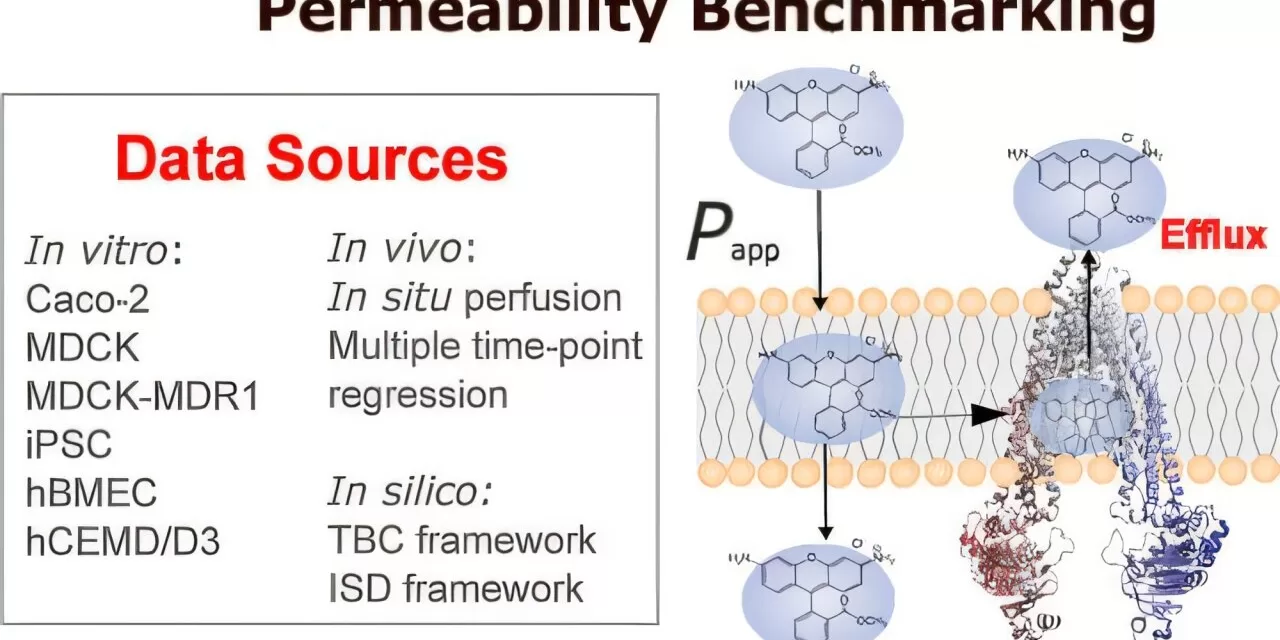
The successful and safe delivery of therapeutics relies on understanding how drugs pass through biological barriers that protect cells. Drug permeability testing is crucial in determining whether medicines can reach their intended targets in the body, but scientists have long struggled to achieve accurate and reproducible data.
There are three key methods for measuring drug delivery success: laboratory testing, animal and human studies, and advanced computer simulations. However, the challenge of reconciling results from these three testing areas remains a persistent issue.
Bridging the Gap
New research, led by the University of Portsmouth in collaboration with the University of Southampton, King’s College London, and the Massachusetts Institute of Technology (MIT), has developed a set of general guidelines to compare values from lab experiments, molecular simulations, and AI. This groundbreaking work is published in the Journal of Chemical Information and Modeling.
Dr. Christian Jorgensen from the University of Portsmouth’s School of Medicine, Pharmacy, and Biomedical Sciences explains, “Drug permeability testing is vital because if a drug can’t cross the right barriers, it won’t work properly, no matter how promising it seems on paper.”
Dr. Jorgensen has spent years trying to compare permeability data from humans, animals, and computer simulations. While each method has its advantages, the goal is to ensure the highest quality drugs make it to market, he says. He adds, “When seeking drug approval, the more evidence you provide on its effectiveness, the more likely it will be approved.”
The study notes that between 2000 and 2015, only 14% of drugs entering clinical trials in the US received FDA approval. Enhanced permeability testing could significantly improve success rates by ensuring drugs reach their targets more effectively, while minimizing side effects.
Testing the Most Complex Systems
The study highlights the importance of understanding drug movement across all biological barriers, including the most complex systems like the blood-brain barrier, which presents a major obstacle for treatments targeting neurological conditions.
“The strength of this research is that we’ve not just tested guidelines against simple barriers in the body—we used one of the most complicated, highly relevant systems for health and therapeutics,” explains Dr. Jorgensen. He believes this work has the potential to accelerate the development of life-saving drugs, especially for brain disorders where drug delivery is notoriously difficult.
The new guidelines offer a roadmap for improving permeability testing and modeling by examining differences in three areas:
- In silico: Computer programs predict how a drug might move through the body based on its chemical properties.
- In vitro: Drugs are tested on cells in controlled lab environments to observe their behavior.
- In vivo: Drugs are tested within living bodies to assess how they work.
Consistency and Collaboration
The study stresses the importance of consistency in experiments, addressing data variability, and adopting FAIR (Findable, Accessible, Interoperable, Reusable) principles for data sharing. The authors also emphasize the need for collaboration across scientific fields to enhance the accuracy of permeability testing and uncover new insights into drug delivery mechanisms.
Professor Martin Ulmschneider from King’s College London adds, “By providing clear benchmarks and recommendations, we aim to unify and refine permeability testing practices, creating a standardized framework that allows scientists to compare their findings reliably.”
This collaboration is set to refine drug delivery testing methods, potentially saving lives by speeding up the approval of effective treatments.
Disclaimer: This article is based on a study led by the University of Portsmouth and its collaborators, as published in the Journal of Chemical Information and Modeling. The views expressed in this article are those of the researchers and do not necessarily reflect the official stance of the institutions involved. For more detailed information, please refer to the original research article: Permeability Benchmarking: Guidelines for Comparing in Silico, in Vitro, and in Vivo Measurements, Christian Jorgensen et al., Journal of Chemical Information and Modeling (2025).