Kolkata, India – In a significant breakthrough, researchers have unveiled a novel computational protocol that promises a more cost-effective approach to managing cholesterol levels. This innovative method, developed through a collaboration between Dr. Suman Chakrabarty’s team at the S. N. Bose National Centre for Basic Sciences and Sarfez Pharmaceuticals, could revolutionize the field of computer-aided drug discovery by targeting harmful protein-protein interactions (PPIs).
Proteins are essential for a wide array of bodily functions, but incorrect protein interactions can lead to diseases, including elevated low-density lipoprotein (LDL) or cholesterol levels. Traditional approaches to inhibit these harmful interactions have faced challenges due to the difficulty of targeting large, smooth PPI sites with small molecule drugs.
Large peptides and antibodies have been used as alternatives, but they come with high costs, storage issues, and the need for injection-based administration. This has driven the pharmaceutical industry to seek more convenient, orally administered small molecules.
The new approach focuses on allosteric inhibitors—drugs that bind to a protein at a site distant from the main interaction interface but still manage to alter the protein’s behavior and prevent harmful interactions. The challenge has been identifying these allosteric sites, which are often transient or hidden.
Dr. Chakrabarty’s team has addressed this problem by developing a computational protocol to predict and identify alternative binding pockets and hotspots on a protein surface that are allosterically coupled to the functional PPI interface. Their findings were recently published in the Journal of Chemical Information and Modeling.
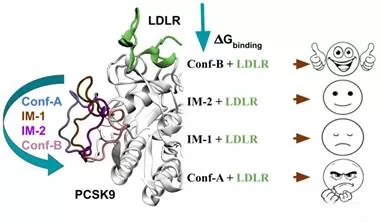
As a test case, the researchers examined the PCSK9 protein, which is crucial in regulating blood cholesterol levels by interacting with the low-density lipoprotein receptor (LDLR). Increased PCSK9-LDLR interaction elevates LDL levels, a key risk factor for heart disease.
Current treatments targeting PCSK9 are effective but expensive and not universally suitable. Therefore, discovering a small-molecule drug that can block the PCSK9-LDLR interaction when taken orally could be transformative.
Dr. Chakrabarty’s team has made notable progress in pinpointing targetable parts of the PCSK9 protein using advanced thermodynamic principles. They propose that by comparing the conformational ensembles of the bound and unbound states of the protein, researchers can identify unique conformations and pockets present in the unbound state for drug targeting. Small molecules that can lock PCSK9 in its unbound state could inhibit its interaction with LDLR, thereby managing cholesterol levels more effectively.
This collaboration between an academic institution and a pharmaceutical company highlights a new paradigm in drug design, emphasizing precision in targeting proteins to prevent diseases. The implications extend beyond cholesterol management, offering a promising pathway for developing treatments for various conditions caused by harmful PPIs.
By leveraging computational simulations and thermodynamic principles, this research represents a significant step forward in the quest for affordable and effective cholesterol-lowering therapies, potentially benefiting millions of people worldwide.