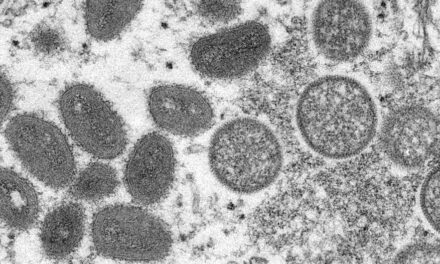
A groundbreaking study conducted by researchers at Johns Hopkins Children’s Center has revealed that infection with a common virus significantly worsens a deadly intestinal disease in premature infants. The research, published in Cellular and Molecular Gastroenterology and Hepatology, suggests that cytomegalovirus (CMV) exacerbates necrotizing enterocolitis (NEC), a severe condition that affects the intestines of preterm babies.
NEC is the most common emergency intestinal complication in premature infants, yet its exact causes remain unclear. The disease, which affects up to 10% of preemies, results in severe inflammation that destroys intestinal tissue. Tragically, about one-third of affected infants do not survive, and treatment options have seen little advancement over the past three decades.
Dr. David Hackam, lead investigator and Garrett Family Professor of Pediatric Surgery at Johns Hopkins University School of Medicine, emphasized the importance of these findings:
“NEC is the most important disease that most people have probably never heard of. By identifying its connection with cytomegalovirus infection, we have now identified an important trigger for NEC, which could save the lives of premature infants who develop this condition.”
Investigating the CMV-NEC Connection
CMV, a virus in the herpes family, is widely prevalent, with 40% to 80% of people worldwide carrying the infection. While typically harmless in healthy individuals, it poses significant risks to unborn babies when transmitted from mother to fetus. Between 30% and 50% of fetuses whose mothers are infected during pregnancy acquire CMV, making it a leading cause of congenital disabilities, including hearing loss.
Dr. Hackam and his team conducted experiments using neonatal mice to model NEC with CMV infection. They observed that infected mice suffered more severe tissue damage and had higher mortality rates than those without CMV. Genetic analysis revealed that CMV activated inflammatory pathways and disrupted metabolism, leading to increased production of an immune protein called toll-like receptor 4 (TLR4).
Potential for New Treatments
Further analysis showed that CMV damaged mitochondria—the cell’s energy factories—by reducing production of adenosine triphosphate (ATP), a crucial energy molecule. When researchers administered adenosine, a common dietary supplement, to infected mice, NEC severity decreased significantly. This suggests that ATP-boosting treatments could potentially mitigate the harmful effects of CMV on NEC.
Additionally, mice genetically modified to lack TLR4 in their intestines exhibited less severe NEC symptoms, further highlighting TLR4 as a potential target for drug development.
Future Implications
While the study offers compelling evidence linking CMV to NEC severity, further research is needed to confirm these findings in human infants. If validated, screening for CMV in pregnant women and preemies, alongside targeted therapies, could transform NEC treatment and improve survival rates.
The research team is now planning additional studies to explore adenosine’s potential as a therapeutic option, as well as other strategies to counteract CMV’s impact on NEC.
Disclaimer
This article is based on scientific research and is intended for informational purposes only. It should not be used as a substitute for professional medical advice, diagnosis, or treatment. Always consult a healthcare provider regarding medical conditions and treatment options.