Researchers at the University of Michigan Health Rogel Cancer Center have developed a groundbreaking urine-based test capable of detecting fragments of DNA released by head and neck tumors. This innovative test holds promise for the early detection of a cancer type that currently lacks reliable screening methods.
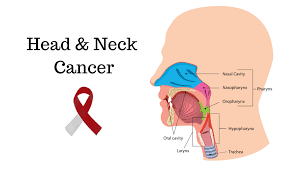
Head and neck cancer, increasingly linked to human papillomavirus (HPV) infection, poses a significant health threat. However, due to the absence of effective screening tools, early detection remains challenging.
Led by Muneesh Tewari, M.D., Ph.D., J. Chad Brenner, Ph.D., and Paul L. Swiecicki, M.D., the research team utilized whole genome sequencing to identify ultra-short DNA fragments released by tumor cells. These fragments, passed into urine through the bloodstream, provide valuable insights into cancer detection.
Chandan Bhambhani, Ph.D., co-first author of the study, emphasized the significance of the findings, highlighting that conventional assays often miss these ultra-short DNA fragments. The team’s unconventional approach led to the development of a urine test specifically tailored for detecting HPV-positive head and neck cancer circulating tumor DNA (ctDNA).
The mail-in test, currently in the discovery phase, has been distributed for research purposes to patients within a hundred-plus miles from Ann Arbor. Participants collect a urine sample and send it back to the U-M laboratory for testing, offering a convenient and non-invasive screening option.
The study’s co-senior author, J. Chad Brenner, Ph.D., noted the test’s potential in detecting cancer recurrences earlier than traditional imaging methods. Encouraged by promising results, the team aims to expand distribution to further validate the test’s efficacy.
While initial studies focused on head and neck cancer, the research also suggests the test’s applicability to other cancer types. The test successfully detected ctDNA in patients with breast cancer and acute myeloid leukemia, hinting at broader diagnostic possibilities.
Bhambhani highlighted the convenience and potential compliance benefits of urine-based testing, particularly for post-treatment follow-up. The ease of self-sample collection could improve patient adherence to surveillance protocols.
Funded by grants from the NIH, NIH/National Cancer Institute, American Cancer Society, and the A. Alfred Taubman Medical Research Institute, this pioneering research represents a significant step forward in cancer detection and underscores the potential of urine-based testing in transforming cancer care.
As the study progresses, researchers remain committed to refining the test’s accuracy and accessibility, offering hope for early detection and improved outcomes in the fight against head and neck cancer and beyond.