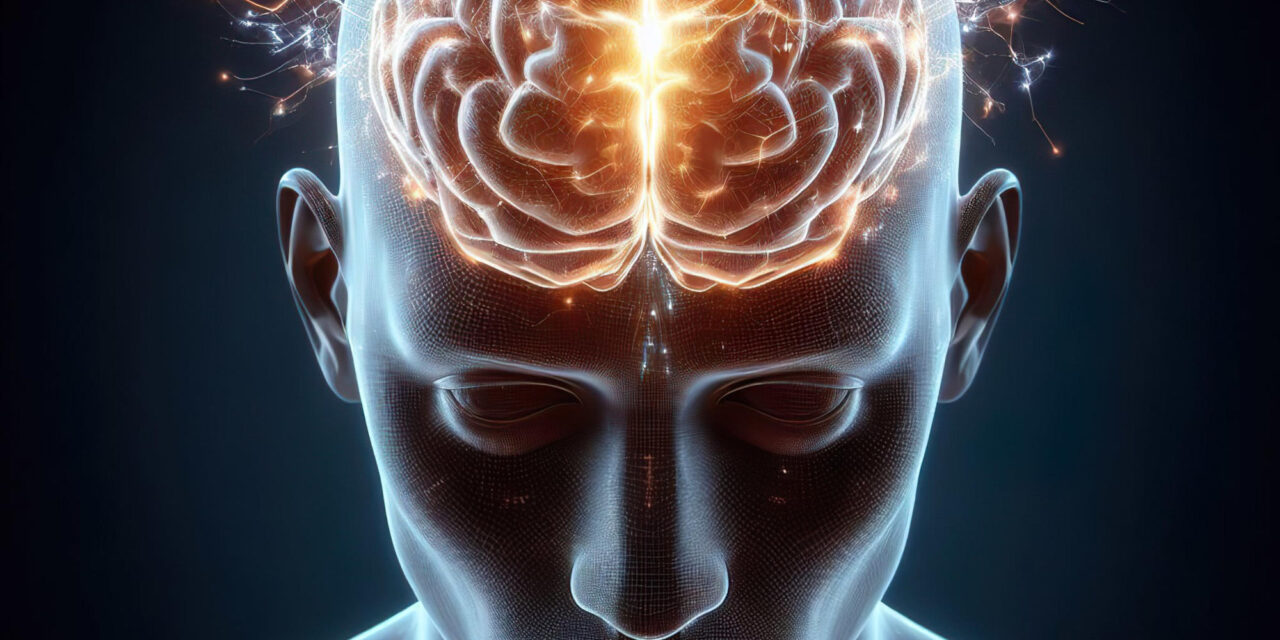
New Insights into Alzheimer’s Origins A groundbreaking study has shed light on the mechanisms driving Alzheimer’s disease, revealing that stalled protein processing in the brain may be a key contributor. Researchers have identified mutations in the presenilin-1 (PSEN1) gene that disrupt the way the gamma-secretase (γ-secretase) enzyme trims amyloid precursor protein (APP), leading to the accumulation of harmful intermediates. This discovery, published as a Reviewed Preprint in eLife, offers a fresh perspective on Alzheimer’s origins and paves the way for innovative treatment approaches.
Examining the Amyloid Cascade Hypothesis For years, the prevailing amyloid cascade hypothesis suggested that a buildup of amyloid beta (Aβ) proteins triggers a cascade of neurodegenerative events, culminating in dementia. However, the new research shifts the focus toward dysfunction in the protein processing system itself.
Lead author Parnian Arafi, Medicinal Chemistry Research Assistant at the University of Kansas, emphasized the need to reassess the role of Aβ in the disease process. “Despite advances in understanding the mutations that lead to Aβ aggregation, uncertainties about the assembly of neurotoxic Aβ proteins remain. Moreover, clinical trials targeting Aβ have shown only modest efficacy, necessitating a broader view of Alzheimer’s pathogenesis,” Arafi stated.
Mutations and Their Impact on APP Processing Senior author Michael Wolfe, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, and his team have previously demonstrated that early-onset familial Alzheimer’s disease (FAD) mutations hinder γ-secretase’s ability to trim APP properly. This leads to the accumulation of longer APP/Aβ intermediates, which may play a critical role in neurodegeneration.
In the current study, the researchers examined six additional mutations associated with FAD, each of which disrupts different steps in Aβ production. The team, collaborating with the Dominantly Inherited Alzheimer Network (DIAN), investigated mutations linked to early-onset cases developing between the ages of 27 and 58.
How Mutations Disrupt the γ-Secretase Process By generating and purifying mutant γ-secretase proteins, the researchers were able to analyze how these mutations affect APP processing. Using mass spectrometry, they measured the protein fragments produced and found that all tested mutations impaired APP trimming at multiple stages.
Further analysis using fluorescently labeled antibodies revealed that these mutations increased the stability of enzyme-substrate complexes, confirming that the proteolytic process had stalled. “We’ve shown that these mutations lead to stalled proteolysis and stabilize the enzyme with its substrate in an intermediate form,” Arafi explained. “This supports our ‘stalled complex’ hypothesis, where these enzyme-substrate complexes, rather than Aβ accumulation, may be the primary drivers of neurodegeneration.”
Implications for Future Alzheimer’s Treatments The study’s findings highlight the need to reconsider current therapeutic strategies for Alzheimer’s disease. Wolfe and his team propose that targeting stalled proteolysis through γ-secretase activators could complement existing treatments that focus on other Alzheimer’s-associated pathways.
“Difficulties in identifying the primary drivers of Alzheimer’s disease have hindered the discovery of effective therapeutics,” Wolfe noted. “By focusing on familial Alzheimer’s disease, we have simplified the identification of pathogenic mechanisms, which could lead to novel treatment strategies.”
Disclaimer: This article is based on ongoing research and is intended for informational purposes only. The findings discussed are part of a preprint study and require further validation through peer-reviewed studies and clinical trials. Readers should consult healthcare professionals for medical advice and treatment options.
Reference: Arafi, P., Devkota, S., Williams, E., Maesako, M., & Wolfe, M. S. (2025). Alzheimer-mutant γ-secretase complexes stall amyloid β-peptide production. eLife. DOI: 10.7554/eLife.102274.2