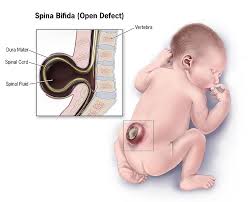
A groundbreaking study led by researchers at the University of California San Diego School of Medicine has shed new light on the underlying causes of spina bifida, the most prevalent structural disorder of the human nervous system.
Led by Keng Ioi Vong, Ph.D., and Sangmoon Lee, M.D. Ph.D., under the guidance of Joseph G. Gleeson, M.D., the team’s investigation has unveiled the first direct association between spina bifida and a common chromosomal microdeletion in humans. Their findings, published in the prestigious journal Science, highlight that individuals harboring this chromosomal deletion, occurring in one out of every 2,500 live births, face a risk of spina bifida more than tenfold higher than the general population.
Spina bifida, also referred to as meningomyelocele, affects approximately one in every 3,000 newborns. Despite its prevalence, the precise triggers behind the condition have largely remained elusive. Gleeson, a prominent figure in neuroscience at UC San Diego, elucidated that while a few mutations had been identified previously, they could only account for a small fraction of the overall risk.
To unravel the genetic underpinnings of the disease, Gleeson’s team collaborated with researchers worldwide to establish the Spina Bifida Sequencing Consortium in 2015. Focusing on a specific microdeletion in chromosome 22, known as 22q11.2del, which has been implicated in various other disorders, they began scrutinizing its presence in spina bifida patients.
“Every patient we recruited displayed the most severe form of spina bifida, and all underwent comprehensive genomic sequencing,” explained Gleeson. “We pinpointed 22q11.2del in 6 out of 715 patients. While this may appear to be a modest percentage, it represents the most prevalent single genetic variation linked to spina bifida.”
Further investigations led the researchers to identify eight additional spina bifida patients carrying the deletion from a cohort of approximately 1,500 individuals recruited due to the presence of the common 22q11.2 deletion.
The team then honed in on the culprit among the numerous genes within the 22q11.2 deletion: a gene called CRKL. Gleeson clarified that while nine other genes resided in this chromosomal region, CRKL emerged as the primary suspect. This conclusion was reinforced when a fortuitous discovery emerged from Dolores Lamb’s team at Weill Cornell College of Medicine, who observed mice lacking Crkl showing signs of spina bifida.
“This finding ignited our excitement, suggesting that CRKL disruption might be a sufficient trigger for spina bifida,” remarked Vong, co-first author of the study. “We corroborated this by removing the mouse Crkl gene ourselves, observing the development of neural tube defects, including spina bifida.”
Turning their attention to potential interventions, the researchers explored the role of folic acid in mitigating CRKL-mediated spina bifida. Prior studies in humans had demonstrated that folic acid supplementation before conception could reduce the incidence of neural tube defects, but the mechanisms remained elusive.
“When we deprived Crkl mutant female mice of folic acid, we observed a significant increase in the prevalence and severity of neural tube defects in their offspring,” noted Vong. “This suggests that folic acid supplementation in pregnant women may not only lower the risk but also mitigate the severity of neural tube defects in their children.”
Gleeson expressed hope that these findings would enhance the understanding of neural tube defect etiology, particularly those associated with common genetic variations like 22q11.2 deletion. Additionally, he anticipates that this research could contribute to promoting healthy pregnancies, improving women’s health, and enhancing outcomes for children affected by spina bifida.
The study involved a collaboration of researchers from UC San Diego School of Medicine Department of Neurosciences, Rady Children’s Institute for Genomic Medicine, Weil Cornell College of Medicine, and the Spina Bifida Sequencing Consortium. Financial support for the study was provided by various entities including the National Institutes of Health, the Howard Hughes Medical Institute, and Rady’s Children Institute for Genomic Medicine.