London, January 5, 2025 — Scientists at the Francis Crick Institute, in collaboration with University College London (UCL) and Imperial College London, have identified a new biochemical pathway central to the progression of inflammatory bowel disease (IBD), paving the way for innovative treatment strategies using existing medications.
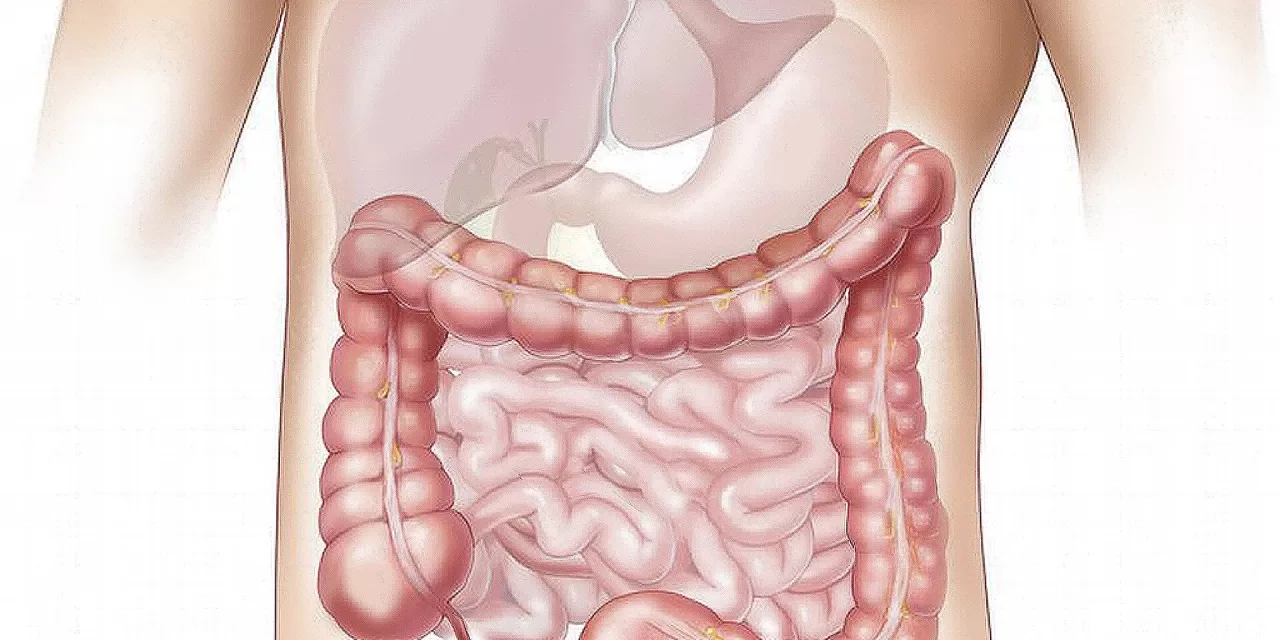
IBD, encompassing Crohn’s disease and ulcerative colitis, affects approximately 5% of the global population and has a particularly high prevalence in the United Kingdom, where one in ten people are diagnosed. By 2022, over half a million individuals in the UK were living with IBD—a sharp increase from earlier estimates of 300,000. Despite advances in treatment, current therapies remain ineffective for many patients, and drug development efforts are frequently stymied by an incomplete understanding of the disease’s underlying causes.
The research team uncovered a previously unexplored ‘gene desert,’ a segment of DNA that does not encode proteins but contains an ‘enhancer’—a regulatory element that modulates the activity of nearby genes. This enhancer, active only in macrophages—a type of immune cell pivotal in IBD—was found to drive the expression of ETS2, a gene linked to an increased risk of the disease. Macrophages, when activated, play a critical role in the inflammation and tissue damage characteristic of IBD.
“Our findings reveal a fundamental mechanism that transforms resting macrophages into inflammatory cells capable of causing the debilitating symptoms of IBD,” said Dr. Emily Harper, lead researcher from the Francis Crick Institute. “By targeting this pathway, we can potentially halt or reverse disease progression.”
In laboratory experiments, the team demonstrated that heightened ETS2 levels in macrophages trigger their transition into inflammatory cells. To counteract this, the researchers are collaborating with LifeArc, a medical research charity, to develop methods for delivering MEK inhibitors—a class of drugs already approved for other conditions—directly to macrophages. These inhibitors indirectly reduce ETS2 activity, offering a targeted approach to managing IBD.
The discovery holds promise for addressing the urgent need for more effective IBD treatments. According to Dr. Harper, the ability to repurpose existing medications could significantly expedite the availability of new therapies, potentially transforming the lives of millions of patients worldwide.
As the prevalence of IBD continues to rise, this groundbreaking research marks a critical step forward in understanding and combating this complex disease. Further studies are underway to refine the therapeutic application of MEK inhibitors and explore additional pathways involved in IBD pathogenesis.