Bethesda, MD – In a groundbreaking study, scientists at the National Institute of Health (NIH) have unveiled significant findings about the behavior of low-density lipoprotein cholesterol (LDL-C), commonly known as “bad” cholesterol. The study, published in Nature, marks the first time researchers have visualized how LDL binds to its receptor in the body—a process crucial for clearing LDL from the bloodstream.
These insights could pave the way for more effective and personalized treatments to lower LDL levels, potentially reducing the risk of cardiovascular disease, the world’s leading cause of death.
“LDL is one of the main drivers of cardiovascular disease, which kills one person every 33 seconds,” said Dr. Alan Remaley, co-senior author of the study and head of the Lipoprotein Metabolism Laboratory at NIH’s National Heart, Lung, and Blood Institute. “If you want to understand your enemy, you want to know what it looks like.”
Until now, the exact mechanism by which LDL binds to its receptor, LDLR, was not clearly understood. Genetic mutations can impair this binding, causing LDL to accumulate in the blood and form plaques in the arteries, leading to atherosclerosis and heart disease.
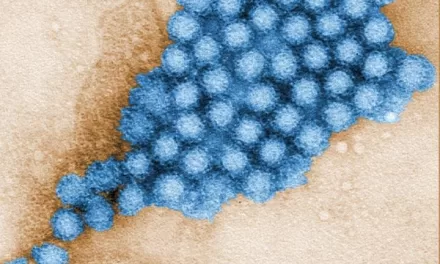
The NIH team utilized advanced cryo-electron microscopy to capture detailed images of the structural protein of LDL when it binds to LDLR. They then used artificial intelligence-driven protein prediction software to model the structure and pinpoint genetic mutations linked to elevated LDL levels.
“LDL is enormous and varies in size, making it very complex,” explained Dr. Joseph Marcotrigiano, co-senior author of the study and chief of the Structural Virology Section at NIH’s National Institute of Allergy and Infectious Diseases. “No one’s ever gotten to the resolution we have. We could see so much detail and start to tease apart how it works in the body.”
The study identified that many mutations affecting the LDL-LDLR binding site are associated with familial hypercholesterolemia (FH), a genetic condition that leads to extremely high LDL levels and early heart attacks. These FH-related mutations were found to cluster in specific regions on LDL.
The findings could lead to the development of targeted therapies to correct these dysfunctional interactions. This could benefit not only individuals with genetic mutations but also those with high cholesterol who are on statins. By precisely understanding the binding mechanism, researchers aim to design new drugs to lower LDL levels more effectively.
For more detailed information, read the original study: Joseph Marcotrigiano, Structure of apolipoprotein B100 bound to the low-density lipoprotein receptor, Nature (2024). DOI: 10.1038/s41586-024-08223-0.
Journal information: Nature.