In a landmark study published in Nature Reviews Microbiology, researchers have reviewed the successes and failures of malaria vaccines, highlighting the potential of the RTS,S and R21 vaccines to significantly reduce malaria-related mortality. With nearly half of the world’s population at risk of malaria, the development of effective vaccines is crucial, especially for vulnerable groups such as pregnant women and young children.
Malaria remains a deadly disease, with over 600,000 fatalities recorded in 2022, a figure that has stagnated since 2015. The disease is caused by five Plasmodium species, with P. falciparum being the most deadly. Despite partial immunity developed over time by females and children, malaria persists due to misdiagnosis, drug resistance, and insecticide-resistant mosquitoes.
Climate Change and Malaria Transmission
The 2023 World Malaria Report emphasizes that climate-induced factors such as increasing temperature, humidity, and rainfall are enhancing parasite and vector multiplication, thereby intensifying malaria transmission and expanding at-risk areas. Additionally, displacement of non-immune groups to endemic regions due to food and economic insecurity is exacerbating the risk of malaria transmission.
The Promise of RTS,S and R21 Vaccines
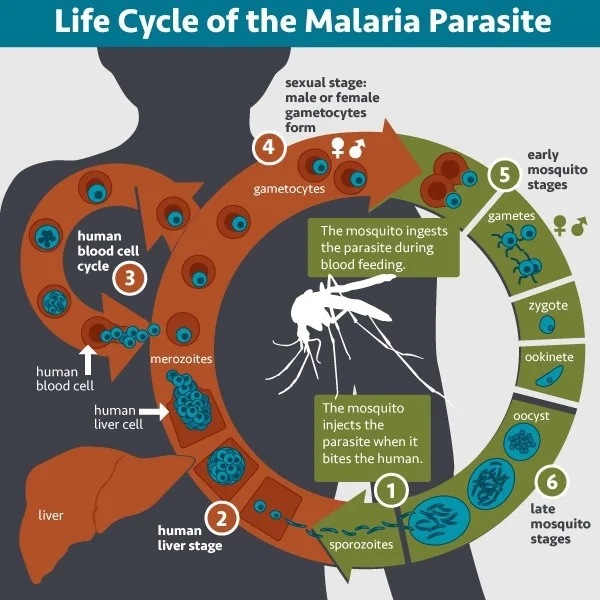
RTS,S and R21 are the first licensed malaria vaccines, targeting the circumsporozoite protein (CSP) of sporozoites. RTS,S, recommended for children in moderate-to-high transmission zones, reduced clinical malaria by approximately 36% over 48 months in phase III trials. R21, approved by Ghana and Nigeria in 2023, showed an even higher efficacy, reducing clinical malaria by 75% with seasonal administration and 68% with standard administration over 12 months.
Vaccine Approaches by Parasite Life Cycle Stage
Malaria vaccine strategies are tailored to different stages of the parasite’s life cycle. Pre-erythrocyte stage vaccines target the liver stages and sporozoites to prevent infection, while blood-stage vaccines focus on merozoite invasion proteins. Despite minimal effectiveness in field trials, some blood-stage vaccine candidates show promise. Transmission-blocking vaccines aim to induce antibodies against mosquito antigens or parasites within mosquitoes.
Limitations and Future Directions
While the achievements of RTS,S and R21 are significant, their protection wanes over time, necessitating annual booster doses. The vaccines’ efficacy can vary based on transmission intensity and timing. The limited supply of saponin extracts used in vaccine adjuvants poses another challenge. Efforts are underway to enhance vaccine efficacy through fractional dosing and combining vaccination with seasonal malaria chemoprevention (SMC).
Addressing Endemic Community Needs
Pregnancy malaria remains a critical issue, leading to high rates of fetal, neonatal, maternal, and infant deaths. Vaccines targeting placental infections, such as those preconceptionally administered, show promise. P. vivax, responsible for a significant proportion of malaria cases outside Africa, presents unique challenges due to its dormant liver stage, requiring innovative vaccine solutions.
Conclusion
The deployment of RTS,S and R21 vaccines on a large scale in Africa is expected to drastically reduce childhood mortality. Combining these vaccines with chemoprevention has already shown a twenty-fold reduction in malaria rates in Mali and Burkina Faso. Ongoing research and new vaccine candidates hold the potential to further enhance the effectiveness of malaria vaccination and broaden its impact.
Journal Reference: Duffy PE, Gorres JP, Healy SA, Fried M. Malaria vaccines: a new era of prevention and control. Nature Reviews Microbiology, 2024, DOI: 10.1038/s41579-024-01065-7, link.