Scientists have identified critical genetic changes that lead to children being born with severely compromised immune systems. In a groundbreaking study, researchers from Newcastle University, the Wellcome Sanger Institute, the Great North Children’s Hospital, and their collaborators have linked mutations in the NUDCD3 gene to Severe Combined Immunodeficiency (SCID) and Omenn syndrome. These rare and life-threatening disorders impair the development of crucial immune cells necessary to fend off infections.
The study, published today in Science Immunology, highlights the potential for early diagnosis and intervention for affected individuals. SCID and Omenn syndrome are genetic disorders that leave children without a functional immune system, making them highly susceptible to life-threatening infections. Without immediate treatments such as stem cell transplants, many affected children do not survive their first year.
While current newborn screening can detect T cell deficiencies, identifying the precise genetic cause enhances diagnostic accuracy and informs curative treatment options. However, for at least 10% of affected families, the genetic cause remains unidentified.
In this study, the research team examined 11 children from four families. Two of the children had SCID, while the other nine had Omenn syndrome. All individuals had inherited mutations that disrupted the function of the NUDCD3 protein, a protein previously not associated with immune function.
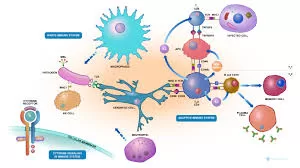
Using patient-derived cells and mouse models, the researchers demonstrated that NUDCD3 mutations interfere with V(D)J recombination, a crucial process for generating diverse T cell receptors and antibodies to combat various pathogens. While mice with the same mutations exhibited milder immune problems, the human patients experienced severe, life-threatening consequences. Remarkably, two patients survived after receiving stem cell transplants, underscoring the importance of early diagnosis and intervention.
Dr. Gosia Trynka, a study author from the Wellcome Sanger Institute and science director at Open Targets, emphasized the importance of early detection. “For babies born with high-risk immunodeficiencies, early detection can mean the difference between life and death. These diseases leave newborns essentially defenceless against pathogens that most of us can easily fend off. The identification of this new disease gene will help clinicians make a prompt molecular diagnosis in affected patients, meaning they can receive life-saving treatments more quickly.”
Professor Sophie Hambleton, the study’s senior author from Newcastle University and a practicing pediatric immunologist at the Great North Children’s Hospital, added: “SCID and Omenn syndrome are devastating disorders requiring complex and timely treatments. The more we understand about their underlying causes, the better we can care for affected babies. Our research aims to fill in the gaps so that families can achieve a molecular diagnosis while we continue learning more about how the immune system functions in health and disease. We are deeply grateful to the families whose invaluable participation in this study will help future generations.”
Notes
- Children with SCID lack T cells needed to combat infections, while those with Omenn syndrome have abnormal T cells that fail to fight infections and attack the body’s own tissues, necessitating urgent treatment.
- Mutations in NUDCD3 prevent the regulation of RAG1, a key enzyme in V(D)J recombination. This results in RAG1 becoming trapped in cell nucleoli instead of facilitating the gene rearrangements that build immune diversity.
This discovery marks a significant step forward in understanding the genetic underpinnings of severe immunodeficiencies and opens new avenues for early diagnosis and effective treatment, offering hope to affected families worldwide.