PHILADELPHIA, PA – Scientists at Thomas Jefferson University have made a significant discovery regarding how the nervous system modulates pain signals, potentially paving the way for novel pain management therapies. Their research, published in the Proceedings of the National Academy of Sciences, sheds light on the molecular mechanisms behind how sensory neurons adapt to repeated stimuli, influencing the intensity of pain sensations.
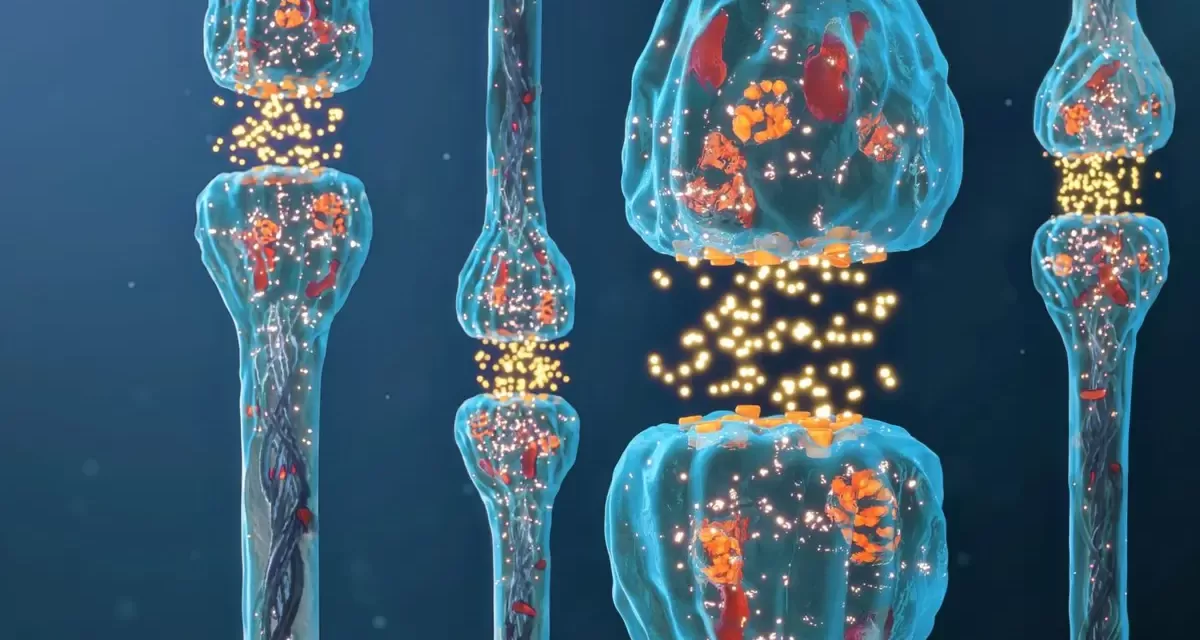
The study focused on the behavior of sensory neurons that respond to temperature, touch, and pain. These neurons communicate through electrical signals called action potentials, which involve the rapid exchange of ions through ion channels. Researchers found that with repeated firing, these action potentials progressively lengthen, a phenomenon observed for years but poorly understood.
The new research reveals that this lengthening is caused by a molecular change in a specific potassium ion channel. This channel, crucial for ending action potentials, relies on phosphate groups for efficient function. When these phosphate groups are insufficient, the channel struggles to close, resulting in prolonged action potentials and heightened pain sensitivity.
“This potassium channel is a major player in ending action potentials, but its function depends on a critical chemical modification,” explained Dr. Manuel Covarrubias, a neuroscientist and senior author of the study.
Through meticulous experimentation, Dr. Covarrubias and his team identified the specific phosphate tagging sites on the potassium channel responsible for these lengthened action potentials. This discovery provides a clear target for future therapeutic interventions.
The implications of this research are significant. By developing treatments that enhance the function of this potassium channel, it may be possible to alleviate chronic pain conditions. This work exemplifies how fundamental research at the molecular level can translate into potential clinical applications.
“This research is a stellar example of how basic research in molecular detail opens the door to potentially novel approaches in clinical translation,” said a spokesperson from the university.
The study, titled “Molecular mechanism governing the plasticity of use-dependent spike broadening in dorsal root ganglion neurons,” was authored by Tyler D. Alexander et al. and published in the Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2411033121.
Disclaimer: This article is based on information provided in the referenced research paper. While the findings are promising, it is important to note that this is basic research and further studies, including clinical trials, are required before these findings can be translated into effective treatments for pain. The information provided in this article should not be interpreted as medical advice. Always consult with a healthcare professional before making any decisions related to your health or treatment.