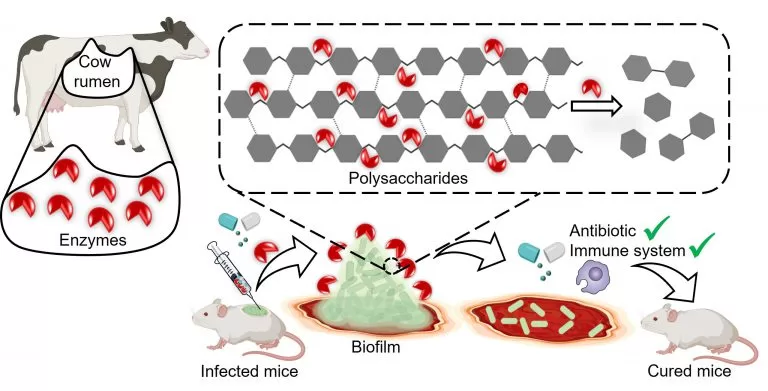
A groundbreaking study from the Indian Institute of Science (IISc) has revealed a promising new approach to combat bacterial infections that are notoriously resistant to antibiotics. The research, published in npj Biofilms and Microbes, demonstrates how an enzyme from the digestive tract of cows can break down the biofilms created by harmful bacteria, making them more susceptible to treatment.
Biofilms are protective barriers formed by bacteria like Klebsiella pneumoniae, which are responsible for a range of hospital-acquired infections, including pneumonia, urinary tract infections, and meningitis. These biofilms are composed of complex layers of sugars, fats, proteins, and DNA, which shield the bacteria from both antibiotics and the body’s immune system.
One of the major challenges in treating Klebsiella pneumoniae infections, especially in diabetic patients, is the biofilm’s ability to protect the bacteria, leading to slow-healing wounds and, in some cases, amputation. The IISc team targeted these biofilms by developing a biocompatible method to degrade the complex polysaccharides that hold the biofilm structure together.
The scientists turned to the bovine gut for inspiration. Cows digest plant matter using microbial enzymes that break down complex polysaccharides, including cellulose and hemicellulose—compounds that bear a striking resemblance to the polysaccharides in bacterial biofilms. By isolating a specific enzyme, called GH-B2, found in the rumen of cows, the researchers were able to synthesize it in the lab.
When tested on four different strains of Klebsiella pneumoniae isolated from hospital patients, GH-B2 successfully dismantled the biofilm in all cases. Surprisingly, the enzyme exhibited broad activity across different bacterial serotypes, even those that vary in how they interact with the immune system.
The breakthrough was not just in breaking down biofilms but in enhancing the effectiveness of antibiotics. When the enzyme was applied to mature biofilms, it made the bacteria 15 times more susceptible to meropenem, a broad-spectrum antibiotic. However, when the enzyme was used early in the biofilm formation process, it prevented the biofilm from developing altogether, making the bacteria 250 times more vulnerable to the antibiotic.
The research team also found that their enzyme improved the immune system’s ability to attack the bacteria. In animal models, the enzyme combined with meropenem completely cleared infected wounds of biofilms, showing promising results for future treatments.
Dr. Debasis Das, Assistant Professor at IISc and corresponding author of the study, highlighted the advantage of this approach: “The chances of the bacteria gaining resistance are very low because our enzyme does not directly harm the bacteria—it simply disrupts the matrix that protects them.”
Looking ahead, the team envisions incorporating this enzyme into practical applications, such as wound dressings for diabetic patients or coatings for medical devices to prevent biofilm formation. “For localized infections, particularly skin infections in diabetic individuals, this could be a game-changer,” said Professor Dipshikha Chakravortty, another corresponding author of the study.
The study underscores the importance of developing new strategies to combat antibiotic-resistant infections, which remain a global health threat. “We need to keep exploring potent enzymes like these to tackle bacterial resistance,” says Chakravortty.
With further research and development, this innovative enzyme could provide a novel therapeutic tool in the fight against stubborn, antibiotic-resistant infections.
Reference: Ramakrishnan, R., Nair, A., Parmar, K., Rajmani, R.S., Chakravortty, D., Das, D. (2024). Combating biofilm-associated Klebsiella pneumoniae infections using a bovine microbial enzyme. npj Biofilms and Microbiomes. Link to study.