San Antonio, Texas — January 2025
The ongoing spread of H5N1 bird flu in the United States is raising alarms among scientists as the virus continues to mutate and infect a growing range of species, including humans. However, a recent study by researchers at the Texas Biomedical Research Institute (Texas Biomed) has offered some relief: FDA-approved antiviral medications remain effective against the virus, even in its mutated forms.
The study, published in Emerging Microbes & Infections, analyzed one of the first human cases of H5N1 in the U.S., which was reported in Texas. This strain showed a distinct set of mutations that enhanced its ability to replicate in human cells and caused more severe disease in mice when compared to a strain isolated from dairy cattle.
Mutations and Risks
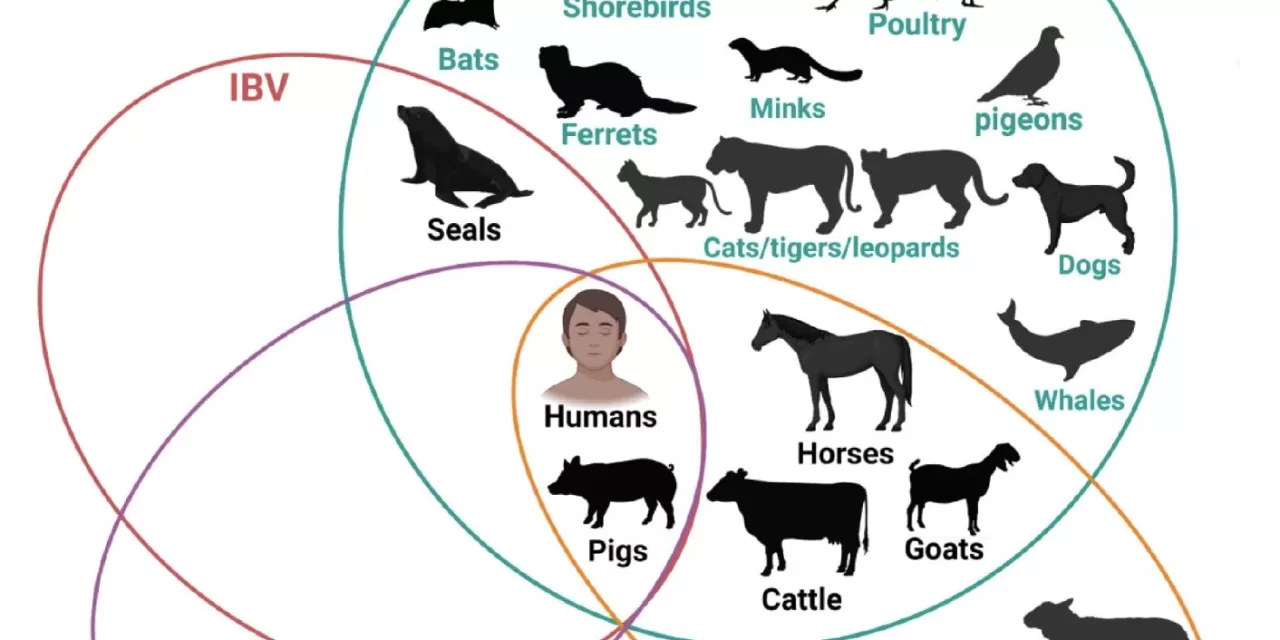
Since its emergence in wild birds and poultry, H5N1 has rapidly expanded its reach, infecting mammals and even dairy cows for the first time in 2024. By early 2025, the outbreak had spread across multiple states, infecting dozens of humans, primarily farm workers. Symptoms in most cases have been mild, such as eye inflammation, and the virus has not yet shown the ability to spread between people. However, the first U.S. death from H5N1 occurred this month following exposure to infected chickens, underscoring the potential dangers.
“The clock is ticking for the virus to evolve to more easily infect and potentially transmit from human to human, which would be a concern,” said Dr. Luis Martinez-Sobrido, a professor at Texas Biomed and influenza expert.
Human vs. Bovine Strains
The Texas Biomed team compared H5N1 strains from a human patient and dairy cattle. They identified nine mutations unique to the human strain, suggesting these changes occurred after the virus jumped from cattle to humans. In experiments using mice, the human strain replicated more efficiently, caused more severe disease, and spread to brain tissue in higher quantities than the bovine strain.
Despite these mutations, laboratory tests showed that FDA-approved antivirals remained effective against both strains. “Fortunately, the mutations did not affect the susceptibility to FDA-approved antivirals,” said Dr. Ahmed Mostafa Elsayed, the study’s lead author.
Next Steps and Preventive Measures
The study emphasized the importance of proactive measures to curb the virus’s spread. The Texas Biomed team is now investigating the specific mutations responsible for the virus’s increased severity and its ability to infect a broad range of species.
“A key priority will be to eradicate bird flu from dairy cows to minimize the risk of further mutations and transmission to humans and other species,” said Dr. Elsayed. He recommended steps such as thorough decontamination of milking equipment and stricter quarantine protocols to eliminate the virus in cattle.
Pandemic Preparedness
With humans having no preexisting immunity to H5N1 and seasonal flu vaccines offering limited protection, antivirals will be crucial if the virus begins spreading between humans. “Antivirals are our first line of defense should a pandemic occur before vaccines are widely available,” Dr. Martinez-Sobrido noted.
The study also highlighted the need for a One Health approach, addressing the virus at the intersection of human, animal, and environmental health to mitigate risks and prevent future outbreaks.
Call for Vigilance
As H5N1 continues to evolve, researchers are urging vigilance and preparedness. “The rapid adaptability of H5N1 underscores the importance of monitoring and swift action to protect both public and animal health,” Dr. Elsayed concluded.
Journal References:
- Ahmed Mostafa et al, Emerging Microbes & Infections, DOI: 10.1080/22221751.2024.2447614
- Ahmed Mostafa et al, mBio, DOI: 10.1128/mbio.02542-24
- Iván Sanz-Muñoz et al, mBio, DOI: 10.1128/mbio.03721-24