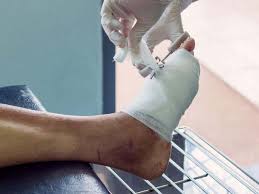
Philadelphia, PA — A groundbreaking study from the Perelman School of Medicine at the University of Pennsylvania has uncovered an unexpected ally in the fight against chronic diabetic wounds. The research, published in Science Advances, highlights the beneficial role of the bacterium Alcaligenes faecalis (A. faecalis) in promoting the healing of hard-to-treat wounds in diabetic patients.
Chronic wounds, such as sores and ulcers that fail to heal or heal very slowly, are a common and debilitating issue for individuals with diabetes. These wounds can be painful, prone to infection, and linked to higher rates of morbidity and mortality. Traditional treatments, including surgical removal of dead tissue and bandaging, have seen limited advancements.
The research team, led by Dr. Elizabeth Grice, the Sandra J. Lazarus Professor in Dermatology, and Ellen K. White, an MD-PhD student, discovered that A. faecalis, a bacterium often found in chronic wounds, could actually facilitate wound healing. Their study revealed that A. faecalis promotes skin cell movements crucial for wound closure by inhibiting enzymes that are excessively produced in diabetic wounds.
“We were initially surprised by the finding that a bacterium could aid in wound healing,” said Dr. Grice. “This discovery motivated us to further explore how A. faecalis could be leveraged to develop new treatments for diabetic wounds.”
The team conducted a series of experiments using diabetic mice, their skin cells, and human diabetic skin samples. They observed that inoculating diabetic mice with A. faecalis led to accelerated wound healing without signs of infection. Additionally, A. faecalis prompted keratinocytes—key cells responsible for wound healing in the skin—to proliferate and migrate more effectively, leading to faster wound closure.
Skin samples from diabetic individuals cultured with A. faecalis showed a statistically significant increase in keratinocyte growth after 10 days. The researchers also noted that A. faecalis treatment influenced gene expression related to immune system activation and downregulated genes responsible for collagen breakdown, particularly matrix metalloproteinases (MMPs), which are elevated in diabetic wounds and hinder healing.
“Matrix metalloproteinases are essential for breaking down cellular connections to allow movement, but their excessive levels in diabetes impede wound healing,” White explained. “Our findings suggest that A. faecalis helps rebalance MMP expression, facilitating faster wound closure.”
This new research opens up exciting possibilities for bacterial-based wound therapies. Future studies will focus on understanding how A. faecalis communicates with skin cells and interacts with other bacteria in the wound environment. There is potential to develop therapeutic strategies that isolate the pro-healing molecules secreted by A. faecalis or target the pathways influenced by its presence.
“Bacterial-based therapies represent a promising frontier in wound care,” said Dr. Grice. “By deepening our understanding of the wound microbiome and its interactions, we may unlock new treatment options for patients suffering from chronic wounds.”
The study was supported by multiple grants from the NIH, including those from the Institute of Nursing Research, National Institute of Arthritis and Musculoskeletal and Skin Diseases, and National Institute of Allergy and Infectious Diseases, among others.