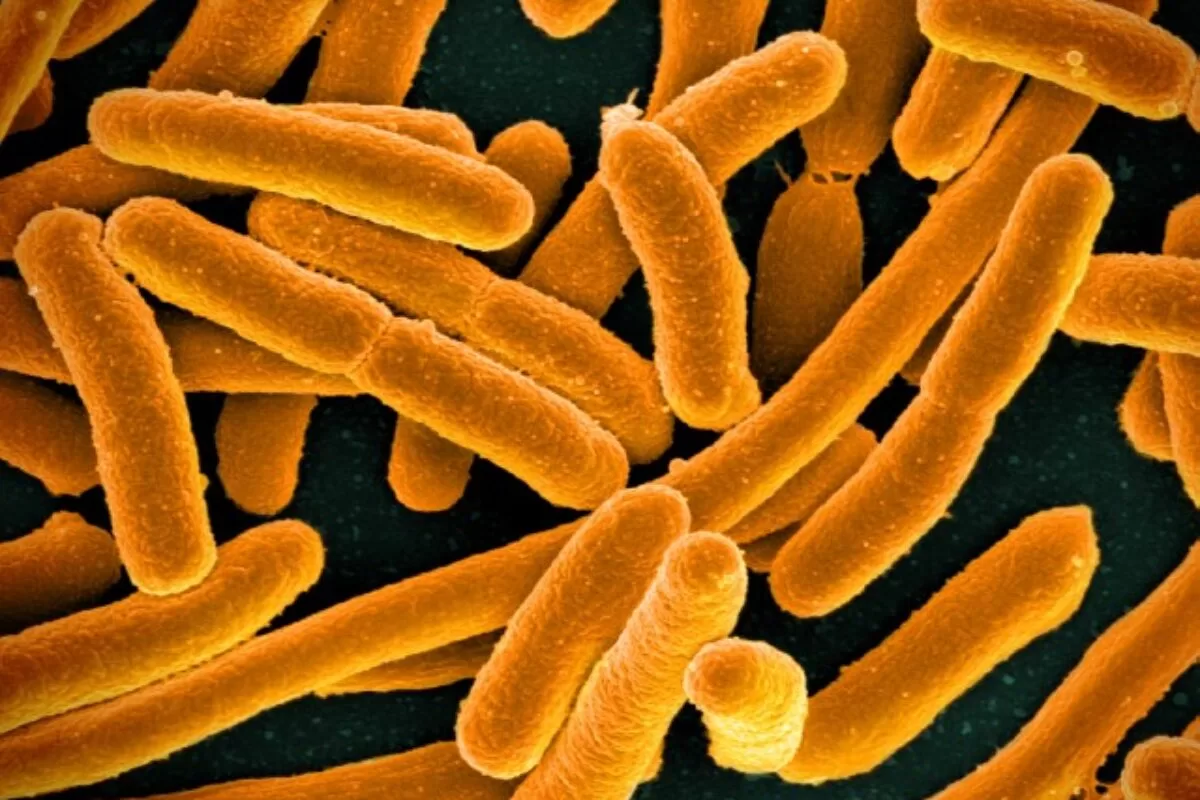
Scientists have made a groundbreaking discovery indicating that bacteria possess memory-like mechanisms governing their strategic behaviors, potentially leading to dangerous infections in humans, such as antibiotic resistance and the formation of bacterial swarms. This newfound understanding holds promise for preventing and combating bacterial infections, particularly those resistant to antibiotics. It revolves around a common chemical element that bacterial cells utilize to encode and transmit these ‘memories’ to subsequent generations.
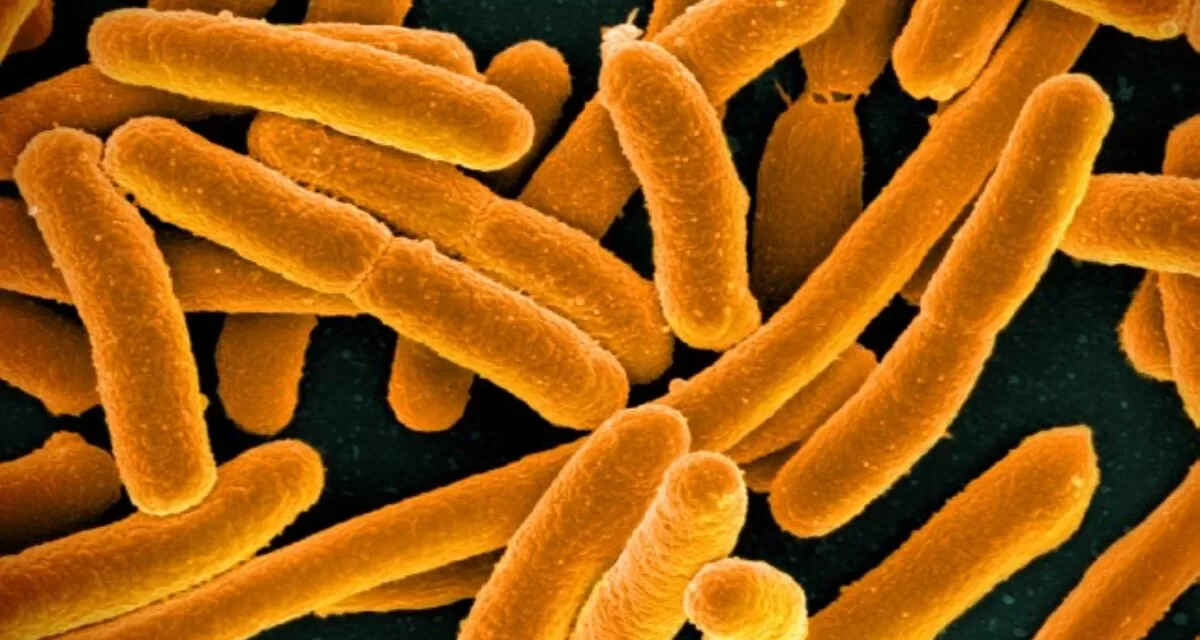
A team from The University of Texas at Austin uncovered that E. coli bacteria employ iron levels as a means of storing information about various behaviors. These stored details can be activated in response to specific stimuli.
Published in the Proceedings of the National Academy of Sciences, the research builds upon previous observations where bacteria showed enhanced collective movements, like swarming, after prior experiences. Seeking the cause behind this phenomenon, the UT-led team delved deeper. Given that bacteria lack neurons or nervous systems, their ‘memories’ don’t resemble reminiscing about childhood events but are more akin to data stored in a computer.
“Although bacteria lack brains, they can assimilate information from their surroundings. If they encounter these environments frequently, they can retain and swiftly access this information for their advantage,” explained Souvik Bhattacharyya, the study’s lead author and a provost early career fellow in the Department of Molecular Biosciences at UT.
The core of this mechanism lies in iron, an abundantly found element on Earth. Individual free-floating bacteria exhibit varying iron levels. The research team noticed that bacterial cells with lower iron levels displayed superior swarming abilities. Conversely, bacteria forming biofilms, dense clusters on solid surfaces, showed higher iron content in their cells. Those bacteria displaying antibiotic tolerance showcased balanced iron levels. These iron-based ‘memories’ persisted for at least four generations before fading away by the seventh generation.
“Before oxygen existed in Earth’s atmosphere, early cellular life relied heavily on iron for various cellular processes. Iron played a pivotal role not only in the origin but also in the evolution of life,” remarked Bhattacharyya. “Therefore, it’s logical that cells would utilize it in this manner.”
The hypothesis posits that when iron levels are low, bacterial ‘memories’ trigger the formation of rapid-moving migratory swarms seeking out iron in the environment. On the contrary, elevated iron levels signal an environment conducive to sticking together and forming biofilms.
“Targeting iron levels presents an avenue for therapeutics, given its critical role in bacterial virulence,” emphasized Bhattacharyya. “Understanding bacterial behavior enhances our capability to combat them effectively.”
Funded by the National Institutes of Health, the study was led by Rasika Harshey, a professor of molecular biosciences and the Mary M. Betzner Morrow Centennial Chair in Microbiology. Nabin Bhattarai, Dylan M. Pfannenstiel, and Brady Wilkins, alongside Abhyudai Singh from the University of Delaware, also contributed to this groundbreaking research.