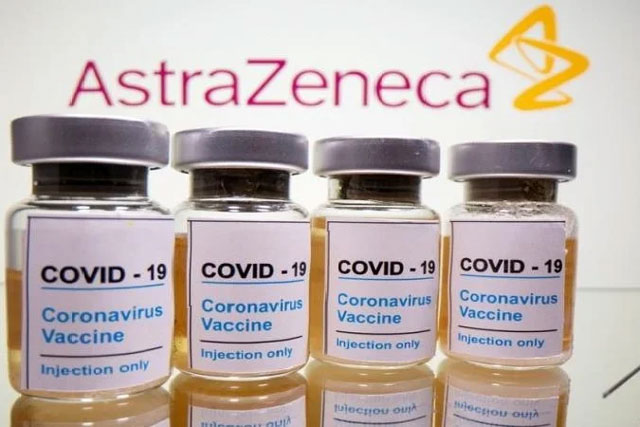
In a significant development, pharmaceutical giant AstraZeneca has taken the decision to recall its Covid-19 vaccine, developed in collaboration with Oxford University, from distribution worldwide. This move comes months after the company acknowledged in a UK court the potential rare side effect of blood clotting associated with the vaccine.
The vaccine, known as Covishield in India and Vaxzevria in Europe, had its “marketing authorisation” voluntarily withdrawn by AstraZeneca. Consequently, it can no longer be utilized within the European Union, as reported by the Telegraph.
Although the company had applied for the withdrawal of the vaccine’s authorization on March 5, it officially took effect on Tuesday.
AstraZeneca, a British-Swedish multinational pharmaceutical company, has conceded in a legal filing to the High Court in February that its Covid-19 vaccine “can, in very rare cases, cause TTS” (Thrombotic Thrombocytopenic Syndrome). This rare side effect is associated with the formation of blood clots and a decreased blood platelet count, which has been linked to at least 81 deaths in the UK, along with numerous serious injuries.
Currently, AstraZeneca is facing legal action from over 50 individuals who claim to be victims or relatives of victims affected by the vaccine’s side effects, in a High Court case in the UK.
However, the pharmaceutical giant has clarified that the withdrawal of the vaccine is due to “commercial reasons” and is “not linked to the court case”. AstraZeneca asserted that “the timing was pure coincidence”.
Explaining the rationale behind the withdrawal, the company cited the surplus availability of updated vaccines due to the emergence of multiple Covid-19 variants and their corresponding vaccines. This surplus has led to a decrease in demand for Vaxzevria, which is no longer being manufactured or supplied. Consequently, AstraZeneca has opted to commence the withdrawal of the marketing authorizations for Vaxzevria within Europe.
AstraZeneca also indicated its intention to collaborate with global regulatory authorities to initiate marketing authorization withdrawals for Vaxzevria in regions where no future commercial demand for the vaccine is anticipated.
The decision to recall the AstraZeneca Covid-19 vaccine underscores the complexities and challenges associated with vaccine development and distribution, especially amidst evolving scientific understanding and public health considerations.