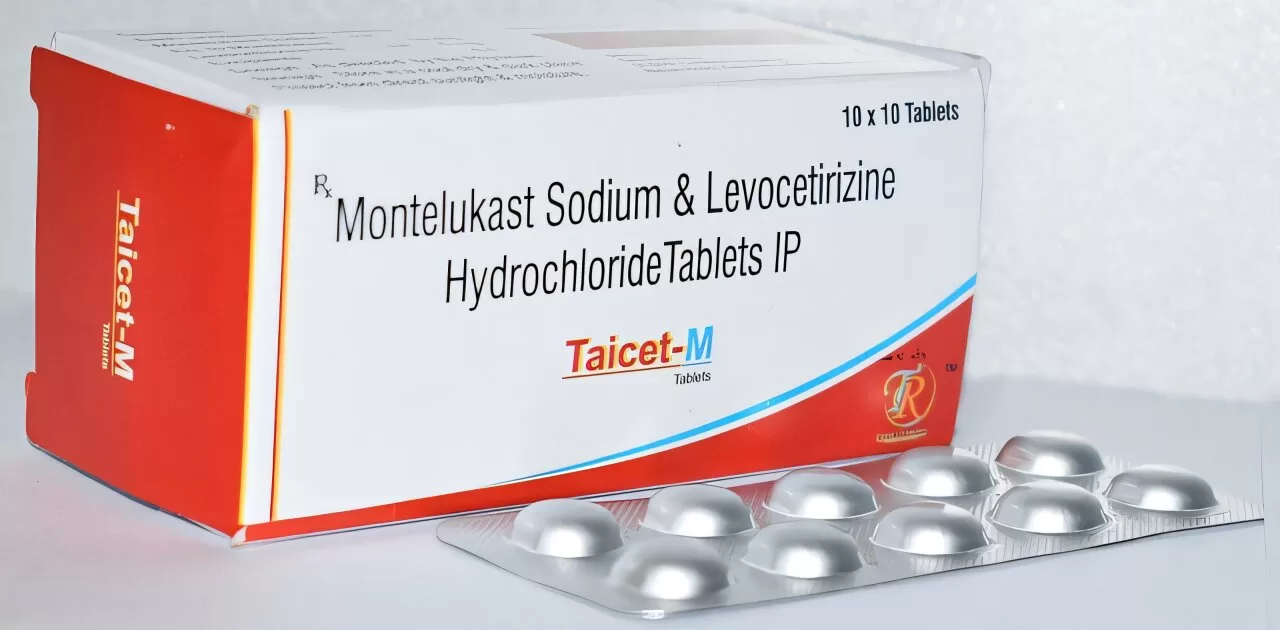
Australia’s Therapeutic Goods Administration (TGA) has issued a safety alert requiring stronger warnings on the asthma and hay fever medication montelukast (brand names: Singulair, Asthakast, Lukafast, Montelair).
The new warnings, presented in a prominent “boxed” format in patient information leaflets, will highlight the potential for serious behavioral and mood-related side effects, including suicidal thoughts and depression.
What is Montelukast?
Montelukast is a prescription medication used to manage mild-to-moderate asthma and seasonal allergies. It works by blocking the action of leukotrienes, chemicals that contribute to airway constriction and mucus production.
The Link to Depression and Suicide:
While rare, studies have shown an association between montelukast use and increased risks of behavioral changes, including:
- Agitation
- Anxiety
- Depression
- Irritability
- Obsessive-compulsive symptoms
- Suicidal thoughts and actions
A recent study of over 100,000 children found a 32% higher incidence of behavioral changes in those taking montelukast compared to those on inhaled corticosteroids alone.
TGA Action:
The TGA’s decision to strengthen warnings aligns with international recommendations and aims to better inform patients and healthcare providers about the potential risks.
Important Note:
If you or your child is taking montelukast, do not stop the medication without consulting your doctor. Sudden discontinuation can increase the risk of an asthma attack.
Discuss your concerns with your doctor. They can assess the risks and benefits of montelukast in your specific situation and explore alternative treatment options if necessary.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Always consult with a qualified healthcare professional for any health concerns.