A research team led by Professor Kevin Tsia, Program Director of the Biomedical Engineering Program at the University of Hong Kong (HKU), has made significant strides in cancer diagnostics with the development of an AI-driven imaging tool that accelerates and refines the diagnostic process, improving medical treatment outcomes for patients.
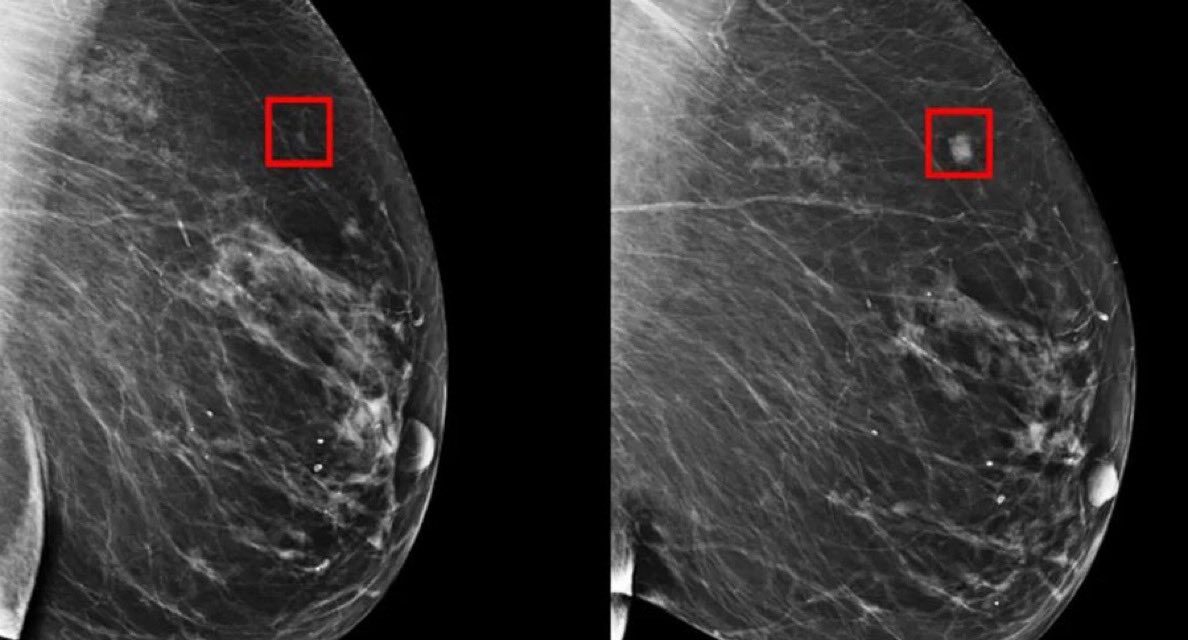
In collaboration with HKU’s Li Ka Shing Faculty of Medicine (HKUMed) and Queen Mary Hospital, the team has successfully implemented Cyto-Morphology Adversarial Distillation (CytoMAD) in the analysis of lung cancer patients and drug tests. The cutting-edge AI method, combined with proprietary microfluidic technology, provides a fast, cost-effective “label-free” imaging solution that allows clinicians to assess tumors at the precision of individual cells. Additionally, CytoMAD aids in determining the risk of metastasis, thus greatly improving the overall accuracy and speed of cancer diagnostics.
CytoMAD employs advanced AI technology to automatically correct imaging inconsistencies, enhance cell images, and extract previously undetectable information, providing healthcare professionals with reliable and accurate data to base their treatment decisions on. This revolutionary technology could transform how cell imaging is analyzed in biomedicine, offering new insights into cell properties and disease progression.
“Until now, there was no cost-effective technique for single-cell imaging due to limitations in scale and clarity. Traditional methods were too slow and not sufficiently informative,” said Professor Tsia. The new AI model addresses these challenges, enabling clearer and more detailed imaging that can be obtained without the need for costly and time-consuming stains or labels.
The study, published in the journal Advanced Science, outlines the breakthrough that allows clinicians to bypass the conventional staining process while preserving the key information required for effective diagnoses. The label-free technology enables quicker results, speeding up the diagnosis and drug discovery process, as patients no longer need to wait long periods for the results.
CytoMAD also addresses the challenge of “batch effects”—unwanted variations that arise from differing experimental conditions, which can hinder accurate biological interpretations. Traditional machine learning models often rely on assumptions about the data, which can reduce their reliability. By contrast, CytoMAD operates without such assumptions, ensuring unbiased cell image analysis.
The new approach isn’t limited to lung cancer, though it remains one of the most prevalent and deadly cancers globally. The tool’s potential extends to the faster screening of drugs and diagnostics, providing valuable insights across a wide range of applications. The technology could also lead to AI-driven predictions for cancer and other diseases, enabling healthcare providers to identify high-risk patients earlier.
Looking ahead, the team plans to apply for funding to conduct clinical trials, focusing on lung cancer patients over a three-year period. The goal is to gather sufficient data to track patient progress and refine the imaging tool for broader clinical use.
Disclaimer: This article is based on the research published by Michelle C.K. Lo et al. in Advanced Science (2024). Results and outcomes referenced are based on preliminary studies and may vary upon further clinical trials and evaluations.