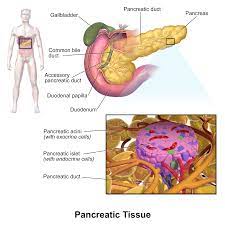
Various cell types within our pancreas play pivotal roles in regulating our blood sugar levels. A gene known as Neurogenin 3 (NEUROG3) is present in pancreatic cells, and mutations in this gene can potentially lead to diabetes. Understanding the behavior and dynamics of this gene, particularly during human development, has remained a puzzle due to its brief activity period during pancreatic growth.
To unravel the mysteries surrounding this gene, scientists from the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden, Germany, and the Novo Nordisk Foundation at the University of Copenhagen, Denmark, employed a unique technique to monitor the gene’s activity and the protein it produces in human pancreas cells. Their innovative approach aimed to establish connections between the dynamic behavior of NEUROG3 and the development of hormone-producing pancreatic cells.
This research could significantly enhance our comprehension of the development of pancreas cells responsible for producing hormones, potentially opening doors to generating more of these cells for therapeutic purposes, such as treating individuals with diabetes.
The pancreas houses distinct cell types that regulate blood sugar levels, including beta cells responsible for insulin production, which aids in lowering blood sugar. When these cells malfunction or die, diabetes can develop. During our body’s growth, all these specialized cells originate from a single cell type in the pancreas known as a pancreatic endocrine progenitor. This cell briefly activates the NEUROG3 gene to carry out its function.
Anne Grapin-Botton’s research group, led by the Managing Director of MPI-CBG in Dresden, partnered with colleagues from the Novo Nordisk Foundation at the University of Copenhagen to delve deeper into these specialized pancreas cells that utilize the NEUROG3 gene and examine how this gene behaves at the single-cell level. They employed specialized markers to visualize NEUROG3 within these cells and monitored their movements through a long-term live imaging method that generated videos. Their investigation revealed that NEUROG3 gene expression levels varied among different cells, with some cells exhibiting high expression and others low expression.
Surprisingly, despite this heterogeneity, all cells with detectable NEUROG3 ultimately transformed into hormone-producing cells. Another unexpected finding was that NEUROG3 operates approximately twice as slowly in humans compared to mice, indicating that it takes more time for the gene to fulfill its role in humans.
The long-term live imaging method enabled the researchers to uncover a process typically concealed within the mother’s womb. The brightness of the cells allowed them to correlate gene activity with cellular behavior. Through this method, the research team also identified another gene, KLK12, as having a role in orchestrating cell movements that initiate the formation of islets of Langerhans when the NEUROG3 gene becomes active.
Anne Grapin-Botton, who oversaw the study, summarized their findings by emphasizing the value of the cell culture systems they developed for understanding fetal organ formation in humans. Their study shed light on how gene activity during fetal development can contribute to diabetes later in life. Importantly, the results suggest flexibility in controlling NEUROG3 when producing endocrine cells for potential therapeutic applications involving the transplantation of these cells into diabetic patients.