In a groundbreaking discovery, scientists at the University of Colorado Anschutz Medical Campus have revealed that immune cells consuming bacteria do not simply store them in specialized compartments, as previously believed. Instead, these cells convert the bacteria into essential nutrients that help build proteins, generate energy, and sustain cellular function.
The findings, published today in the journal Nature, challenge long-held assumptions about immune cell behavior and open new avenues for understanding inflammation and disease.
“We are what we eat,” said Dr. Angelo D’Alessandro, Ph.D., professor of biochemistry and molecular genetics at the University of Colorado School of Medicine and co-senior author of the study. “What we eat changes our composition, and when immune cells consume bacteria, the same transformation occurs within them.”
The Role of Macrophages in Nutrient Recycling
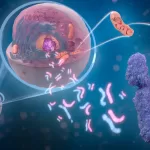
The study focused on macrophages, immune cells responsible for engulfing and digesting bacteria. Researchers discovered that when macrophages consume live bacteria, the process triggers an inflammatory response. However, consuming dead bacteria does not induce inflammation.
“When phagocytic cells eat dead bacteria, some of the small molecules they recycle signal them not to induce inflammation, suggesting everything is under control,” D’Alessandro explained. “But when they consume live bacteria, this calming signal is absent, leading to an inflammatory reaction that contributes to various diseases.”
The scientists investigated the metabolic pathways involved and identified a crucial protein complex, mTORC1, which regulates how macrophages process nutrients from bacteria. Additionally, they found that dead bacteria contain a molecule called cAMP, which signals immune cells that the bacteria are no longer a threat, allowing for better metabolic control and reduced inflammation.
Implications for Disease and Antibiotic Resistance
Chronic inflammation plays a role in numerous health conditions, including cancer, long COVID, chronic fatigue syndrome, and shingles. By understanding the mechanisms that switch inflammation on and off, researchers aim to develop therapies to better control immune responses.
“Over the next decade, we will face increasing challenges with antibiotic-resistant bacteria,” D’Alessandro noted. “Understanding the natural regulatory mechanisms of the immune system can help us either suppress or enhance these responses as needed.”
The study was conducted in collaboration with researchers from CU Anschutz, Charité–Universitätsmedizin Berlin in Germany, and the University of Bordeaux in France. Co-authors include Assistant Research Professor Julie A. Reisz Haines, Ph.D., Parnika Mukherjee, Ph.D., Juliette Lesbats, Ph.D., and Johan Garaude, Ph.D.
For more details, the full study can be accessed in Nature at: www.nature.com/articles/s41586-025-08629-4.
Disclaimer: This article is for informational purposes only and is based on published scientific research. It should not be considered medical advice. Consult a healthcare professional for medical concerns or treatment options.