Houston, TX – A groundbreaking study from researchers at Rice University has introduced a novel theoretical framework to better understand menopause timing. Published in the Biophysical Journal, the study applies stochastic analysis, a mathematical approach that evaluates potential outcomes using random probability, to examine the complex dynamics of ovarian aging.
Led by Anatoly Kolomeisky, professor of chemistry and chemical and biomolecular engineering, the research team developed a model that quantitatively predicts menopause timing. By analyzing how ovarian follicles transition through different stages, the study explains why menopause occurs and highlights individual variability and cross-population differences. These findings could have significant implications for fertility planning, hormonal therapy decisions, and age-related health risks associated with ovarian aging.
“By considering menopause as a sequential process involving random transitions of follicles, we can better understand individual variability and population-wide trends in menopause timing,” Kolomeisky explained.
A Theoretical Breakthrough in Ovarian Aging
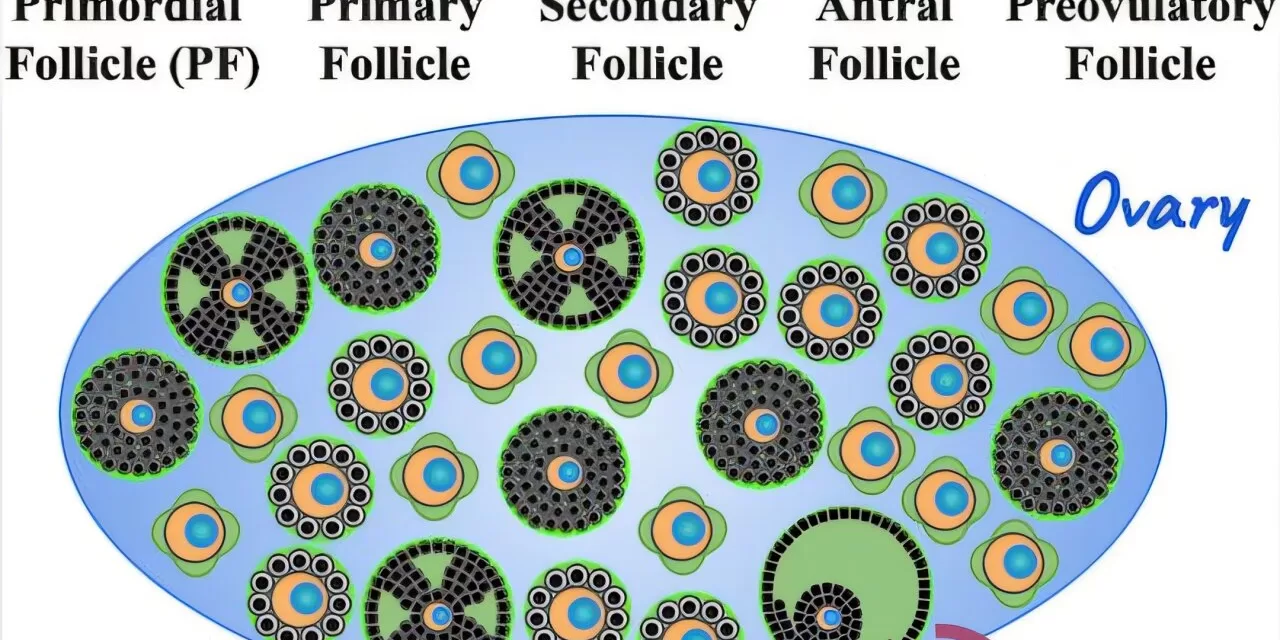
The study hypothesizes that ovarian aging follows a stochastic sequential process influenced by follicles transitioning through multiple developmental stages. Unlike previous research that focused primarily on hormonal and genetic factors, this study incorporates extensive computer simulations and analytical calculations to model ovarian follicle depletion with greater precision.
Through this approach, researchers were able to align their theoretical predictions with medical data from diverse populations, offering a detailed quantitative framework for menopause timing.
“By applying stochastic analysis, we can move beyond broad observations and develop precise, predictive insights into menopause timing and variability,” Kolomeisky added.
Key Insights Into Menopause Timing
The study uncovered a universal relationship among three key factors: the initial ovarian follicle reserve, the rate of follicular depletion, and the threshold that triggers menopause. A particularly surprising finding was the synchronization of follicular transitions, which may play a role in regulating the timing of menopause.
“One of the most unexpected findings was the synchronization of follicular transitions, which may regulate the timing of menopause,” Kolomeisky noted. “This suggests that underlying biochemical processes ensure a relatively consistent age of menopause despite individual variations.”
The study was co-authored by Anupam Mondal, a postdoctoral fellow at the Center for Theoretical Biological Physics, and Evelina Tcherniak, an undergraduate student in the Department of Biomolecular Engineering.
The full study, titled Stochastic Analysis of Human Ovarian Aging and Menopause Timing, is available in the Biophysical Journal.
Disclaimer:
This article is for informational purposes only and does not constitute medical advice. The findings discussed are based on theoretical modeling and should not be used as a substitute for professional medical consultation. Readers are encouraged to seek guidance from healthcare professionals regarding menopause and related health concerns.