New York, NY – February 26, 2025 – In a historic medical breakthrough, Towana Looney, the longest-living recipient of a genetically engineered pig kidney, has successfully completed her recovery and returned home to Alabama. Looney, 53, underwent the pioneering xenotransplant procedure at NYU Langone Health on November 25, 2024, and has since been under the close observation of the NYU Langone Transplant Institute team.
“I feel blessed,” Looney shared. “I’m so grateful to be alive and thankful to have received this incredible gift. It couldn’t have happened without God and the amazing team of doctors, nurses, and researchers who have been by my side.”
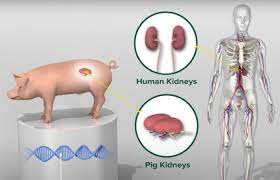
Looney’s procedure involved a kidney from a 10-gene-edited pig, making her only the third person in the world to receive such a transplant. After spending three months in New York for postoperative care, she returned home on February 25, though she will continue to be monitored with monthly checkups at NYU Langone.
“Towana’s recovery from a long history of kidney failure and dialysis treatment has been nothing short of remarkable,” said Dr. Robert Montgomery, who led the procedure and serves as director of the NYU Langone Transplant Institute. “We are committed to giving her the best possible chance at life and providing ongoing care from our world-class team of experts. We’re so pleased to see her go back home to her extended family healthy and ready to take on a whole new phase in her long life ahead.”
A Groundbreaking Xenotransplant
Looney’s health had been deteriorating due to eight years of dialysis for end-stage kidney failure. Her condition was further complicated by a previous kidney donation to her mother and a pregnancy-related complication that led to unusually high levels of harmful antibodies, preventing her from receiving a human transplant.
The xenotransplant was made possible through the work of Dr. Jayme Locke, a transplant surgeon and innovator in xenotransplantation. Dr. Locke, now a faculty member at NYU Langone, initiated a compassionate use application with the U.S. Food and Drug Administration (FDA) to allow Looney to receive the experimental treatment.
The kidney, known as a UKidney, was provided by Revivicor Inc., a subsidiary of United Therapeutics Corporation. It was developed with 10 gene edits to enhance compatibility and reduce the likelihood of rejection. No unapproved devices or medications were used in Looney’s surgery or postoperative care.
Future of Xenotransplantation
Earlier this month, the FDA authorized United Therapeutics to launch the first-ever multicenter clinical study of the UKidney, starting with six kidney failure patients and potentially expanding to 50 participants.
The need for such innovations is pressing. Nearly 104,000 people are currently on the U.S. transplant waiting list, with over 90,400 awaiting a kidney. According to the Centers for Disease Control and Prevention, approximately 35.5 million adults in the U.S. suffer from chronic kidney disease, and nearly 808,000 have end-stage kidney disease. However, only 27,759 kidney transplants were performed in 2024.
Looney’s case represents a significant milestone in the field of xenotransplantation, offering hope to thousands in need of lifesaving organ transplants.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. The xenotransplant procedure remains experimental, and its long-term effects are still being studied. Consult a healthcare professional for medical guidance related to organ transplantation and treatment options.