Hong Kong’s Breakthrough in Leukemia Treatment
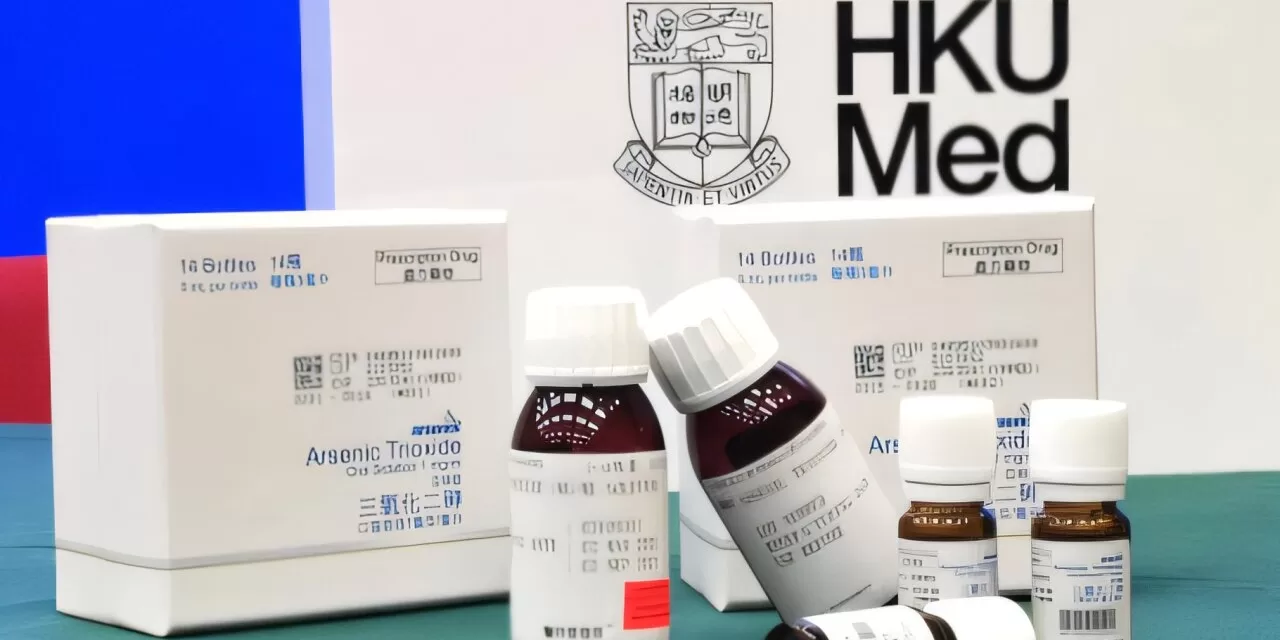
Researchers at the LKS Faculty of Medicine of the University of Hong Kong (HKUMed) have developed a groundbreaking oral formulation of arsenic trioxide (Oral-ATO; ARSENOL) for the treatment of acute promyelocytic leukemia (APL), a type of blood cancer that once had a high fatality rate. This innovation marks a historic milestone as the first prescription medicine wholly invented and manufactured in Hong Kong, securing patents in the U.S., Europe, and Japan.
Clinical Success of Oral-ATO
Following over two decades of dedicated research, HKUMed successfully integrated Oral-ATO into APL treatment plans. Clinical studies demonstrated an impressive overall survival (OS) rate exceeding 97%, significantly reducing treatment burdens and side effects. A 15-year study involving more than 400 patients showed a 100% molecular remission rate and an 80% five-year OS rate, without the need for bone marrow transplantation.
Further studies led to its incorporation into frontline treatment, yielding a 100% leukemia-free survival (LFS) and OS rate at five years. This advancement has drastically reduced early deaths associated with APL complications, once as high as 30%.
Oral-ATO: A New Standard in APL Treatment
Dr. Harinder Gill, Clinical Associate Professor at HKUMed and lead investigator, emphasized that Oral-ATO combined with all-trans retinoic acid (ATRA) and ascorbic acid (AAA) provides a highly effective and safe regimen. The completely oral treatment is feasible for outpatient settings and minimizes the need for chemotherapy across all risk levels, including children and adults.
International Recognition and Expansion
Oral-ATO has earned orphan drug designation (ODD) from the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), alongside an investigational new drug (IND) designation from the FDA. The APL Asian Consortium, spearheaded by HKUMed, has validated the superiority of this regimen in Hong Kong, Malaysia, Singapore, and Taiwan.
Global Clinical Applications
- Asia & Greater Bay Area (GBA): Oral-ATO has been approved by the Guangdong Provincial Medical Products Administration (GDMPA) for clinical use at HKU-Shenzhen Hospital. Research collaborations continue in Singapore, Malaysia, and Taiwan.
- United Kingdom: A nationwide Phase 3 study, supported by Blood Cancer UK and led by the University of Cardiff, will evaluate Oral-ATO in frontline APL management.
- U.S. & Europe: HKUMed researchers and industry partners will initiate clinical studies in North America and Europe, following regulatory approvals.
Transforming APL into a Curable Disease
Dr. Gill expressed pride in translating this research into a globally accessible treatment. “Oral-ATO is a game-changer for APL patients, offering a convenient and effective therapy that enhances their quality of life. Our mission is to ensure universal access to this life-saving treatment, making APL a curable disease.”
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Patients should consult healthcare professionals for diagnosis and treatment options. The effectiveness of Oral-ATO may vary based on individual conditions, and regulatory approvals may differ across regions.