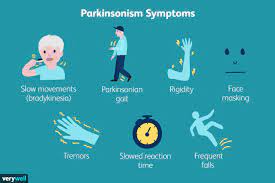
Washington, D.C. – The U.S. Food and Drug Administration (FDA) has approved Onapgo (apomorphine hydrochloride) injection as the first and only subcutaneous apomorphine infusion device for the treatment of motor fluctuations in patients with advanced Parkinson’s disease. This milestone approval was announced by Supernus Pharmaceuticals in a recent news release.
Onapgo is a wearable subcutaneous infusion device that provides continuous treatment during waking hours, offering more consistent control of motor fluctuations. The device is expected to be available in the second quarter of 2025 and will be introduced with a strong support network, including a specialized nurse education program.
Clinical Efficacy and Safety
The FDA’s approval was based on data from a phase 3, 12-week, multicenter, parallel-group, double-blind, randomized, placebo-controlled study involving 107 patients. Results showed that, compared to the placebo group, patients using Onapgo experienced a significant reduction in daily OFF time (2.6 hours versus 0.9 hours) and an increase in GOOD ON time (2.8 hours versus 1.1 hours). These improvements were noticeable as early as the first week of treatment and remained consistent throughout the study period.
Commonly reported adverse events (≥10% incidence) included infusion-site nodules, nausea, excessive sleepiness, infusion-site skin irritation, headache, and insomnia.
Expert Reactions
Andrea Merriam, CEO of the Parkinson & Movement Disorder Alliance in Phoenix, highlighted the impact of motor fluctuations on Parkinson’s patients. “As the motor symptoms of Parkinson’s disease worsen over time, patients report alternating states between ON when their medication is working, and OFF when it’s not working optimally,” Merriam stated. “These on-again, off-again changes are disruptive and can happen at any time, which is why consistent daily control of OFF time is key to improving how patients feel and move. For many, continuous treatment options like Onapgo can help to make days with Parkinson’s more predictable.”
Approval of Onapgo was granted to Supernus Pharmaceuticals.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Patients should consult their healthcare providers before considering any new treatment options. The safety and efficacy of Onapgo may vary based on individual conditions. Always seek professional medical guidance for Parkinson’s disease management.