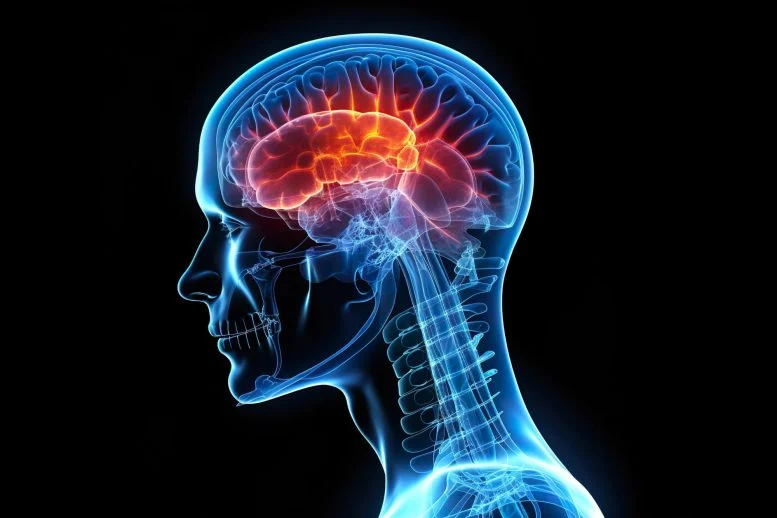
A groundbreaking study led by Dr. Peter Canoll’s lab at Columbia University, in collaboration with the Columbia University Irving Medical Center, Zuckerman Institute, and Irving Institute for Cancer Dynamics, provides fresh insights into how gliomas—aggressive brain tumors—disrupt brain function, causing debilitating neurological symptoms such as seizures and cognitive impairments. The research, published in Neuron, uncovers mechanisms that could lead to rapid therapeutic interventions for these life-threatening conditions.
Gliomas, known for their aggressive nature, infiltrate the brain’s cortex, disrupting neuronal function and triggering hyperexcitability. The study reveals that this disruption leads to severe neurological symptoms, including seizures and cognitive difficulties. Researchers employed innovative techniques, such as in-vivo two-photon imaging, to investigate neuronal changes in a glioma mouse model. This cutting-edge technology enabled precise, real-time mapping of brain activity and structural changes at the neuronal level.
The findings show that glioma-associated neurons lose critical synaptic connections and become hyperexcitable, triggering epileptic discharges in response to normal sensory stimuli. These alterations are particularly concerning for patients who experience severe cognitive and physical challenges.
In a remarkable breakthrough, the study also highlights the potential of the experimental drug AZD8055, which targets the mTOR signaling pathway. Within just six hours of treatment, AZD8055 was able to reverse these disruptive neuronal changes, offering a promising therapeutic approach to mitigate the effects of gliomas on brain function.
“This study not only highlights the mechanisms driving glioma-induced dysfunction but also shows that key aspects of this damage are rapidly reversible,” said Dr. Canoll. “Our novel experimental mouse model offers a powerful tool for studying the effects of neuronal activity on glioma growth and provides a means to directly stimulate activity in the tumor and monitor its effects on both tumor cells and neurons.”
The study’s innovative approach paves the way for future research, including the use of the serial two-photon tomography system available at the Zuckerman Institute and IICD. This system promises to provide unparalleled imaging precision, allowing researchers to explore how tumor cells interact with neuronal structures at the tumor’s infiltrative margin.
Dr. Canoll emphasized that future research would focus on deepening the understanding of how tumor cells and neurons interact, potentially opening up new therapeutic strategies to combat gliomas.
This study highlights the power of interdisciplinary collaboration, combining expertise from pathology, neurosurgery, neurology, systems biology, and advanced imaging technologies. The work represents a significant step toward understanding the neurological complications caused by gliomas and offers hope for developing treatments that could improve patient outcomes.
Disclaimer: This article is based on a study published in Neuron. While the findings are promising, further clinical trials and research are needed to confirm the effectiveness and safety of the experimental treatment AZD8055 for human patients. The research mentioned is still in its early stages and has only been tested in a mouse model. Always consult medical professionals for advice on health-related matters.