Tuberculosis (TB) continues to be one of the leading infectious disease killers worldwide, with drug-resistant forms of the disease presenting an ongoing challenge. However, a significant breakthrough has emerged from an international clinical trial, which has identified three new, safe, and effective drug regimens for treating rifampin-resistant tuberculosis, one of the most concerning forms of drug-resistant TB.
The findings were published in the New England Journal of Medicine and represent a major step forward in the fight against TB. The research was led by Harvard Medical School, with contributions from the endTB project, a collaboration between Partners In Health, Médecins Sans Frontières, and Interactive Research and Development, along with global academic and clinical partners.
New Drug Regimens: A Hopeful Advancement
The newly discovered regimens use recently approved drugs to enhance treatment options, providing physicians with new ways to shorten treatment durations, reduce side effects, and eliminate the need for daily injections. These regimens also offer critical alternatives for patients facing drug intolerance, medication shortages, or further resistance to drugs.
The endTB clinical trial, which began in 2017, tested five new all-oral 9-month regimens. These regimens combined two recently discovered TB drugs—bedaquiline and delamanid—with older, established medications. These two drugs, introduced to the market in 2012-2013, were the first new TB treatments in nearly half a century. Notably, a third drug, pretomanid, was not included in the trials but was later granted emergency FDA authorization for use in highly drug-resistant TB in 2019.
Impressive Trial Results
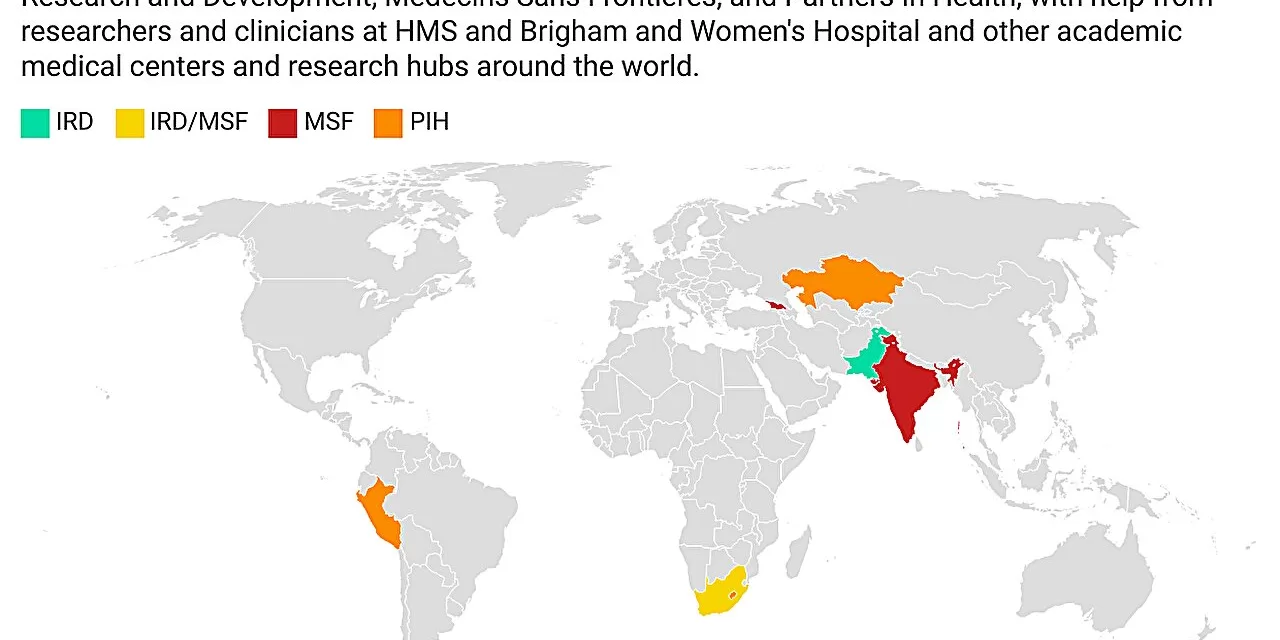
The trial, which enrolled 754 patients across seven countries—Georgia, India, Kazakhstan, Lesotho, Pakistan, Peru, and South Africa—revealed that the new regimens were successful for 85-90% of patients, compared to an 81% success rate for the control group. The control group received a standard of care treatment that also included newly developed drugs.
These findings offer a promising new direction for treating rifampin-resistant and multidrug-resistant TB (MDR-TB), which affects an estimated 410,000 individuals each year. Despite progress, only 40% of these patients are diagnosed and treated, and only 65% of those receive successful treatment outcomes.
Inclusive and Accessible Treatment
The study included a diverse population, including children, people with HIV or hepatitis C, and pregnant women—groups typically excluded from clinical trials. This inclusion represents a significant advancement, ensuring that more vulnerable populations will benefit from these new treatment options.
In August 2024, the World Health Organization (WHO) added the three successful regimens from the endTB trial to the list of recommended treatments for rifampin-resistant and multidrug-resistant TB. The WHO’s endorsement ensures that these new regimens will be available to neglected groups, including pregnant women.
The affordability of these new treatment options is another key achievement. Following efforts to end patent exclusivity for bedaquiline, the cost of two of the regimens is now below $500—an access goal set by activists more than a decade ago.
A Transformative Step for TB Treatment
Co-principal investigator Carole Mitnick, professor of global health and social medicine at Harvard Medical School and director of research for the endTB project, emphasized the transformative impact of these findings. “This Harvard-led partnership among NGOs, ministries of health, and academic partners identified three new regimens that will make lifesaving care dramatically more accessible,” Mitnick said.
The endTB trial marks a crucial turning point in the global effort to end TB, providing more effective, less toxic, and shorter treatment options for those suffering from drug-resistant forms of the disease.
Disclaimer: The information provided in this article is based on the study published in the New England Journal of Medicine. The effectiveness of the new regimens is subject to further research and clinical validation. Always consult with healthcare professionals for advice on the treatment of drug-resistant tuberculosis.